
”¾ĢāÄæ”æŌ×Ó½į¹¹ÓėŌŖĖŲÖÜĘŚ±ķ”¢ŌŖĖŲŠŌÖŹČżÕß¹ŲĻµĆÜĒŠ”£
A”¢B”¢D”¢E”¢FĪŖŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄĒ°ĖÄÖÜĘŚŌŖĖŲ£¬ĘäÖŠAµÄ×īĶā²ćµē×ÓŹżŹĒĘäÄŚ²ćµē×ÓŹżµÄ2±¶£¬B”¢D”¢EĪŖĶ¬ÖÜĘŚŌŖĖŲ£¬BŌ×ÓµÄŗĖĶāµē×Ó×ÜŹżŹĒĘäĪ“³É¶Ōµē×ÓŹżµÄ5±¶£¬EŌ×Ó×īĶā²ćÓŠ1øöĪ“³É¶Ōµē×Ó£¬FŌ×ÓŗĖĶāÓŠ22ÖÖŌĖ¶ÆדĢ¬µÄµē×Ó”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©FŌŖĖŲĪ»ÓŚÖÜĘŚ±ķ_____________Ēų£¬Ęä¼Ūµē×ÓÅŲ¼Ķ¼ĪŖ£ŗ_____________”£
£Ø2£©B”¢D”¢EČżÖÖŌŖĖŲÖŠ£¬µŚŅ»µēĄėÄÜ×īŠ”µÄŹĒ_____________ (ĢīŌŖĖŲ·ūŗÅ)£»Š“³öAD2µÄµČµē×ÓĢå_____________ (·Ö×ÓŗĶŅõĄė×Óø÷Š“Ņ»ÖÖ)”£
£Ø3£©AO2ŗĶDO2ČŪµćøߵďĒ_____________£¬ŌŅņŹĒ_____________”£
”¾“š°ø”æ d ![]() S CO2 »ņN2O, CNO- »ņSCN- SO2 SO2”¢CO2 ¾łĪŖ·Ö×Ó¾§Ģå,SO2 Ļą¶Ō·Ö×ÓÖŹĮæ½Ļ“óĒŅĪŖ¼«ŠŌ·Ö×Ó,·¶µĀ»ŖĮ¦“ó
S CO2 »ņN2O, CNO- »ņSCN- SO2 SO2”¢CO2 ¾łĪŖ·Ö×Ó¾§Ģå,SO2 Ļą¶Ō·Ö×ÓÖŹĮæ½Ļ“óĒŅĪŖ¼«ŠŌ·Ö×Ó,·¶µĀ»ŖĮ¦“ó
”¾½āĪö”æA”¢B”¢D”¢E”¢FĪŖŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄĒ°ĖÄÖÜĘŚŌŖĖŲ£¬AµÄ×īĶā²ćµē×ÓŹżŹĒĘäÄŚ²ćµē×ÓŹżµÄ2±¶£¬ĪŖCŌŖĖŲ£¬B”¢D”¢EĪŖĶ¬ÖÜĘŚŌŖĖŲ£¬BŌ×ÓµÄŗĖĶāµē×Ó×ÜŹżŹĒĘäĪ“³É¶Ōµē×ÓŹżµÄ5±¶£¬ŌņBµÄŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ1s22s22p63s23p3£¬ĖłŅŌBĪŖPŌŖĖŲ£¬EŌ×Ó×īĶā²ćÓŠ1øöĪ“³É¶Ōµē×Ó£¬ŌņEĪŖClŌŖĖŲ£¬DÖ»ÄÜĪŖSŌŖĖŲ£¬FŌ×ÓŗĖĶāÓŠ22ÖÖŌĖ¶ÆדĢ¬µÄµē×Ó£¬ĖµĆ÷FŌ×ÓŗĖĶāÓŠ22øöµē×Ó£¬ŌņFĪŖTiŌŖĖŲ”£
(1). FĪŖTiŌŖĖŲ£¬Ī»ÓŚÖÜĘŚ±ķÖŠµŚ4ÖÜĘŚ£¬µŚIVB×壬ŌŚÖÜĘŚ±ķÖŠŹōÓŚdĒųŌŖĖŲ£¬Ęä¼Ūµē×ÓÅŲ¼Ź½ĪŖ3d24s2£¬Ōņ¼Ūµē×ÓÅŲ¼Ķ¼ĪŖ![]() £¬¹Ź“š°øĪŖ£ŗd£ŗ
£¬¹Ź“š°øĪŖ£ŗd£ŗ![]() £»
£»
(2). Ķ¬ÖÜĘŚÖ÷×åŌŖĖŲ£¬Ėę×ÅŌ×ÓŠņŹżµÄŌö“󣬵ŚŅ»µēĄėÄܳŹĻÖŌö“óµÄĒ÷ŹĘ£¬µ«ŹĒµŚ¢ņA×åŗĶµŚVA×åŌŖĖŲ·“³££¬ĖłŅŌP”¢S”¢ClČżÖÖŌŖĖŲÖŠ£¬µŚŅ»µēĄėÄÜ×īŠ”µÄŹĒS£¬µČµē×ÓĢåŹĒÖøŌ×ÓøöŹżĻąĶ¬£¬¼Ūµē×Ó×ÜŹżĻąĶ¬µÄĪ¢Į££¬ŌņÓėCS2»„ĪŖµČµē×ÓĢåµÄÓŠ£ŗCO2»ņN2O£¬CNO£»ņSCN££¬¹Ź“š°øĪŖ£ŗS£»CO2»ņN2O£¬CNO£»ņSCN££»
£Ø3£©AO2ĪŖCO2£¬DO2ĪŖSO2£¬¶žÕß¾łĪŖ·Ö×Ó¾§Ģ壬Ļą¶Ō·Ö×ÓÖŹĮæŌ½“󣬷Ö×Ó¼ä×÷ÓĆĮ¦Ō½“ó£¬ČŪ·ŠµćŌ½øߣ¬ĖłŅŌČŪµćøߵďĒSO2£¬¹Ź“š°øĪŖ£ŗSO2£»SO2”¢CO2¾łĪŖ·Ö×Ó¾§Ģ壬SO2Ļą¶Ō·Ö×ÓÖŹĮæ½Ļ“óĒŅĪŖ¼«ŠŌ·Ö×Ó£¬·¶µĀ»ŖĮ¦“ó”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æøł¾ŻĶ¼µÄÄÜĮæĶ¼£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£ŗ
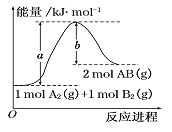
A. A2(g) + B2(g) = 2AB(g) ŹĒŅ»øö·ÅČČ·“Ó¦
B. 2molABµÄ×ÜÄÜĮæ“óÓŚ1molA2ŗĶlmolB2µÄÄÜĮæÖ®ŗĶ
C. 2AB(g) = A2(l) + B2(l) ”÷H<(b-a)kJ/mo1
D. 1molA2(g)ŗĶ1molB2(g)µÄÄÜĮæÖ®ŗĶĪŖakJ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓÉ14COŗĶ12CO×é³ÉµÄ»ģŗĻĘųĢåÓėĶ¬ĪĀĶ¬Ń¹ĻĀæÕĘųµÄĆܶČĻąµČ(æÕĘųµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæĪŖ29£©£¬ŌņĻĀĮŠ¹ŲĻµÕżČ·µÄŹĒ
A. »ģŗĻĘųĢåÖŠ£¬12COÕ¼ÓŠµÄĢå»żµČÓŚ14COÕ¼ÓŠµÄĢå»ż
B. »ģŗĻĘųĢåÖŠ£¬12COÓė14CO·Ö×ÓøöŹżÖ®±ČĪŖ1”Ć2
C. »ģŗĻĘųĢåÖŠ£¬12COÓė14COÖŹĮæÖ®±ČĪŖ15”Ć14
D. »ģŗĻĘųĢåÖŠ£¬12COÓė14COĆܶČÖ®±ČĪŖ14”Ć15
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ( )
A. “ÓCH4”¢NH4£«”¢SO42£ĪŖÕżĖÄĆęĢå½į¹¹£¬æÉĶĘ²āPH4£«”¢PO43£Ņ²ĪŖÕżĖÄĆęĢå½į¹¹
B. 1molĢ¼»Æ¹č¾§ĢåÖŠ£¬Ę½¾łŗ¬ÓŠ4mol C”ŖSi¹²¼Ū¼ü
C. Ė®µÄ·Šµć±ČĮņ»ÆĒāµÄøߣ¬ŹĒŅņĪŖH2O·Ö×Ó¼ä“ęŌŚĒā¼ü£¬H2S·Ö×Ӽ䲻ÄÜŠĪ³ÉĒā¼ü
D. ijĘųĢ¬ĶÅ“Ų·Ö×Ó½į¹¹ČēĶ¼ĖłŹ¾£¬øĆĘųĢ¬ĶÅ“Ų·Ö×ӵķÖ×ÓŹ½ĪŖEF»ņFE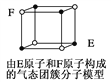
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĪŖĮĖĪ¬³ÖÉśĆüŗĶ½”æµ£¬ČĖŅŖ“ÓŹ³ĪļÖŠÉćČ”ÓŖŃųĖŲ£¬ĻĀĮŠĪļÖŹÖŠ²»ŹōÓŚÓŖŃųĖŲµÄŹĒ£Ø £©
A. ĘĻĢŃĢĒB. Ź³“×C. Ė®D. Ö„ĀéÓĶ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀ±ķĪŖŌŖĖŲÖÜĘŚ±ķÖŠĒ°ĖÄÖÜĘŚµÄ²æ·ÖŌŖĖŲ£¬±ķÖŠĖłĮŠµÄ×ÖÄø·Ö±š“ś±ķŅ»ÖÖ»ÆѧŌŖĖŲ£¬øł¾ŻŅŖĒó»Ų“šĻĀĮŠø÷Š”Ģā£ŗ
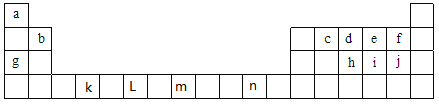
£Ø1£©¢ŁŌŖĖŲ·Ē½šŹōŠŌĒæČõ±Č½ĻÓŠŗܶą·½·Ø£¬ĘäÖŠfŗĶjµÄ·Ē½šŹōŠŌĒæČõµÄŃŠ¾æ·½°øÖŠ²»æÉŠŠµÄŹĒ_________£ØĢīŠņŗÅ£©
a.±Č½ĻĮ½ÖÖµ„ÖŹµÄŃÕÉ« b.±Č½ĻĒā»ÆĪļµÄĪČ¶ØŠŌ c.ŅĄ¾ŻĮ½ŌŖĖŲŌŚÖÜĘŚ±ķµÄĪ»ÖĆ
d.±Č½ĻµēøŗŠŌ e.±Č½Ļ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®ŗĻĪļµÄĖįŠŌ
¢Śøł¾ŻŌŖĖŲŌ×ÓµÄĶāĪ§µē×ÓÅŲ¼µÄĢŲÕ÷£¬æɽ«ŌŖĖŲÖÜĘŚ±ķĒ°ĖÄÖÜĘŚŌŖĖŲ·Ö³É4øöĒųÓņ£¬·Ö±šĪŖsĒų”¢pĒų”¢dĒų”¢dsĒų£¬ŌņŹōÓŚsĒųµÄŌŖĖŲÓŠ_______ÖÖ£¬ŹōÓŚdĒųµÄŌŖĖŲÓŠ_______ÖÖ£»ŌŖĖŲnŹōÓŚ________Ēų”£
¢ŪŌŚc”¢d”¢eČżÖÖŌŖĖŲÖŠ£¬µēøŗŠŌÓÉŠ”µ½“óµÄĖ³ŠņŹĒ______________,µŚŅ»µēĄėÄÜÓɓ󵽊”µÄĖ³ŠņŹĒ____________(ÓĆŌŖĖŲ·ūŗÅ»Ų“š)”£
£Ø2£©¢ŁŠ“³ö n2£«µÄŗĖĶāµē×ÓÅŲ¼Ź½£ŗ______________________”£
¢ŚŠ“³ökŌŖĖŲ»łĢ¬Ō×ӵļŪµē×ÓÅŲ¼Ź½£ŗ_____________”£
¢ŪŠ“³ö LŌŖĖŲ»łĢ¬Ō×ÓµÄĶāĪ§µē×ÓÅŲ¼Ź½£ŗ_____________”£
¢ÜŠ“³ömŌŖĖŲ»łĢ¬Ō×Ó¼Ūµē×ӵĹģµĄ±ķŹ¾Ź½£ŗ________________________£¬øĆŌŖĖŲŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆĪŖ£ŗ__________________________”£
¢Żj¼ņµ„ŅõĄė×ӵĽį¹¹Ź¾ŅāĶ¼ĪŖ____________”£
£Ø3£©¢ŁŌŖĖŲiµÄĒā»ÆĪļ·Ö×ÓŹōÓŚ____________£ØĢī”°¼«ŠŌ”±»ņ”°·Ē¼«ŠŌ”±£©·Ö×Ó£¬Ęä·Ö×ÓµÄæռ乹ŠĶĪŖ____________£¬øĆĒā»ÆĪļ·Ö×ÓÖŠiŌ×Ó¹ģµĄµÄŌÓ»ÆĄąŠĶŹĒ__________£» ÓÉiÓėeŠĪ³ÉµÄie42£Ąė×Ó£¬Ęäæռ乹ŠĶĪŖ__________(ÓĆĪÄ×ÖĆčŹö)”£
¢ŚŅŃÖŖcd- Óė d2 ½į¹¹ĻąĖĘ£¬1 mol cd- ÖŠ![]() ¼üŹżÄæĪŖ___________£¬ÓÉdŠĪ³ÉµÄĄė×Ód3£ÓėCO2»„ĪŖµČµē×ÓĢ壬Ōņd3£µÄ·Ö×Ó¹¹ŠĶĪŖ___________”£
¼üŹżÄæĪŖ___________£¬ÓÉdŠĪ³ÉµÄĄė×Ód3£ÓėCO2»„ĪŖµČµē×ÓĢ壬Ōņd3£µÄ·Ö×Ó¹¹ŠĶĪŖ___________”£
¢Ūf2ĶØČėĻ”NaOHČÜŅŗÖŠæÉÉś³ÉOf2,Of2·Ö×Ó¹¹ŠĶĪŖ___________£¬ĘäÖŠŃõŌ×ÓµÄŌӻƷ½Ź½ĪŖ_______£»
¢Ü»ÆŗĻĪļj2eµÄĮ¢Ģå¹¹ŠĶĪŖ_________£¬ÖŠŠÄŌ×ӵļŪ²ćµē×Ó¶ŌŹżĪŖ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠŹµŃé²Ł×÷»ņŹĀŹµÓėŌ¤ĘŚŹµŃéÄæµÄ»ņĖłµĆ½įĀŪ¶ŌÓ¦ÕżČ·µÄŹĒ(””””)
Ń”Ļī | ŹµŃé²Ł×÷»ņŹĀŹµ | ŹµŃéÄæµÄ»ņ½įĀŪ |
A | µ»ĘÉ«ŹŌŅŗ | ĖµĆ÷ŌČÜŅŗÖŠŅ»¶Øŗ¬ĖłÓŠFeCl3 |
B | CaO | ÓĆÉśŹÆ»ŅÖʱøNaOHČÜŅŗ |
C | ŠĀŹÕ¼ÆµÄĖįÓź | ĖįÓźÖŠŅ»¶Øŗ¬ÓŠSO42- |
D | H3PO3+2NaOH(×ćĮæ)=Na2HPO3+2H2O | H3PO3ŹōÓŚČżŌŖĖį |
A. A B. B C. C D. D
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓŠĻĀĮŠ×°ÖĆĶ¼
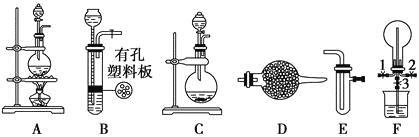
¢ń.Ģ½¾æĀČĘųÓė°±ĘųµÄ·“Ó¦
(1)ĪŖÖĘČ”øÉŌļ°±Ęų£¬æɽ«×°ÖĆCÓė____________(Ģī×°ÖƱąŗÅ)Į¬½Ó£»×°ÖĆCÖŠµÄÉÕĘæÄŚ¹ĢĢåŅĖŃ”ÓĆ________________________”£
a£®¼īŹÆ»Ņ b£®ĀČ»ÆøĘ c£®ĪåŃõ»Æ¶žĮ× d£®ÅØĮņĖį
(2)×°ÖĆA”¢E”¢EĮ¬½ÓæÉÖĘČ”“æ¾»”¢øÉŌļµÄĀČĘų£¬AÖŠ·¢ÉśµÄĄė×Ó·“Ó¦·½³ĢŹ½ĪŖ£ŗ_________________£»ŌņĮ½øöE×°ÖĆÄŚµÄŅ©Ę·ŅĄ“ĪŹĒ_________”¢_____________”£
¢ņ.Ģ½¾æijŠ©ĪļÖŹµÄŠŌÖŹ
(3)ĄūÓĆ×°ÖĆA”¢E£¬æÉÉč¼ĘŹµŃé±Č½ĻCl£ŗĶBr£µÄ»¹ŌŠŌĒæČõ£¬ÄÜÖ¤Ć÷½įĀŪµÄŹµŃéĻÖĻó_______________”£
(4)ČōĄūÓĆ×°ÖĆA”¢E½ųŠŠŅŅĻ©ÓėäåĖ®·“Ó¦µÄŹµŃ飬Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½_________________”£
(5)½«×°ÖĆB”¢C·Ö±šÓėFĻąĮ¬ŗ󣬽ųŠŠH2SÓėSO2·“Ó¦µÄŹµŃ锣FµÄÉÕĘæÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________________£»FµÄÉÕ±ĖłĘšµÄ×÷ÓĆŹĒ_______________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĢ¼ĖįÄʵÄĪļÖŹĄą±šŹĒ£Ø £©
A.ĖįB.¼īC.ŃĪD.ĖįŠŌŃõ»ÆĪļ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com