
| �ζ����� | NaOH��Һ�����mL�� | |
| V1 | V2 | |
| 1 | 3.05 | 44 |
| 2 | 1.45 | 41.5 |
| 3 | 7.65 | 47.6 |
| c(��)?c(��) |
| c(����) |
| 40.05mL+39.95mL |
| 2 |
| c(��)?c(��) |
| c(����) |
| 0.1000mol/L��0.04L |
| 0.02L |


 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ͭ��Һ������������Һ��Ӧ��Ba2++SO42-=BaSO4�� |
| B���ô����ܽ�ˮ���е�Mg��OH��2��2H++Mg��OH��2=Mg2++2H2O |
| C����ҵ���ð�ˮ���ն�������2OH-+SO2=SO32-+H2O |
| D����NaOH��Һ�м�������Ca��HCO3��2��Һ��Ca2++2HCO3-+2OH -=CaCO3��+CO32-+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��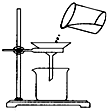 ��Һ���з�������ܵĹ������� |
B��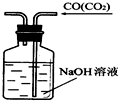 ��ȥCO�����е�CO2���� |
C��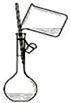 ������ƿ��ת��Һ�� |
D��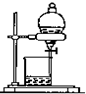 ���뻥�����ܵ�����Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
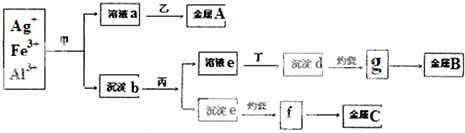
| A�������������ƣ���Ϊϡ���� |
| B������bΪ������Ҫ�ɷ�Ϊ����d�ͳ���e |
| C��g��f��Ϊ�������ҵ�Ͼ����õ��g��f��ö�Ӧ�������� |
| D������A��B��C�ֱ�ΪAg��Fe��Al |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��̽�ն�Ա��-���ᣬ��С���߽��˻�ѧ�Թ�����֪�����߳�������Ϊ�Թ������ࡰ���˵�Ұ�ޡ������������ᷴӦ�����ʻ�����ˮ��Һ�����������ܿ����ǣ��������ͨ����
��̽�ն�Ա��-���ᣬ��С���߽��˻�ѧ�Թ�����֪�����߳�������Ϊ�Թ������ࡰ���˵�Ұ�ޡ������������ᷴӦ�����ʻ�����ˮ��Һ�����������ܿ����ǣ��������ͨ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���Ȼ��� | B��̼������ |
| C������þ | D��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��IBr��˫ԭ�ӷ��� |
| B���ںܶ෴Ӧ�У�IBr��ǿ������ |
| C����NaOH��Һ��Ӧ����NaBr��NaIO |
| D����ˮ��Ӧʱ���������������ǻ�ԭ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com