
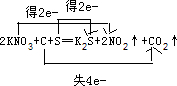 £¬¹Ź“š°øĪŖ£ŗKNO3”¢S£»C£»
£¬¹Ź“š°øĪŖ£ŗKNO3”¢S£»C£»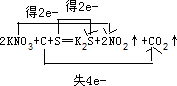 £»
£»


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģ¹óÖŻŹ”øßŅ»ÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŃ”ŌńĢā
»šŅ©ŹĒÖŠ¹śµÄ”°ĖÄ“ó·¢Ć÷”±Ö®Ņ»£¬ÓĄŌ¶ÖµµĆŃ×»Ę×ÓĖļ½¾°Į£¬Ņ²ÓĄŌ¶»į¼¤Ąų×ÅĪŅĆĒČ„·Ü·¢Ķ¼Ē攣ŗŚ»šŅ©ŌŚ±¬ÕØŹ±£¬·¢ÉśČēĻĀ·“Ó¦£ŗS£«2KNO3+3C£½K2S+N2”ü+3CO2”ü£¬ĘäÖŠŃõ»Æ¼ĮĪŖ£Ø £©
A£®S B£® C C£® KNO3 D£® S ”¢KNO3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ½ĖÕŹ”ŠģÖŻŹŠøßŅ»£ØÉĻ£©ĘŚÖŠ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com