
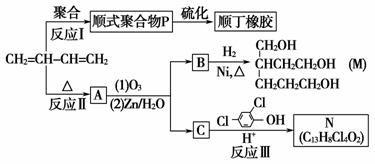
˳�����Ʊ�������֬��ԭ��M�Լ�ɱ����N�ĺϳ�·�����£�
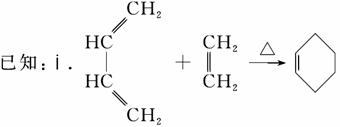
��.RCH==CHR�� RCHO��R��CHO(R��R�������������)
RCHO��R��CHO(R��R�������������)
(1)CH2==CH��CH==CH2��������________��
(2)��Ӧ��ķ�Ӧ������(ѡ����ĸ)________��
a. �Ӿ۷�Ӧ b�����۷�Ӧ
(3)˳ʽ�ۺ���P�Ľṹ��ʽ��(ѡ����ĸ)________��
a.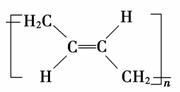
b.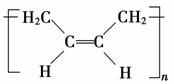
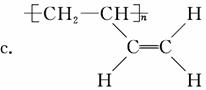
(4)A����Է�������Ϊ108��
�ٷ�Ӧ��Ļ�ѧ����ʽ��_____________________________________��
��1 mol B��ȫת����M�����ĵ�H2��������________g��
(5)��Ӧ��Ļ�ѧ����ʽ��_________________________________��
(6)A��ijЩͬ���칹������ͬ�ķ�Ӧ������Ҳ������B��C��д������һ��ͬ���칹��Ľṹ��ʽ__________________��
������(1)CH2==CH��CH==CH2������Ϊ1,3������ϩ��(2)��CH2==CH��CH==CH2�ۺ�����˳ʽ�ۺ���P�ķ�ӦΪ�Ӿ۷�Ӧ��(3)CH2==CH��CH==CH2��˳ʽΪ�Ӿ۷�Ӧ�õ�˳ʽ�ۺ���P����˵���ۺ���P�к���̼̼˫������CH2==CH��CH==CH2��1,4���ӳɷ�ʽ���мӳɣ��þۺ���PΪCH2��CH==CH��CH2��˳ʽ�ṹΪ����CH2ԭ������̼̼˫����ͬ�࣬��Ϊ ��(4)����A����Է�������Ϊ108,108��14��8ȱ4������A�ķ���ʽC8H12�������Ϣi��֪����Ӧ��Ϊ
��(4)����A����Է�������Ϊ108,108��14��8ȱ4������A�ķ���ʽC8H12�������Ϣi��֪����Ӧ��Ϊ
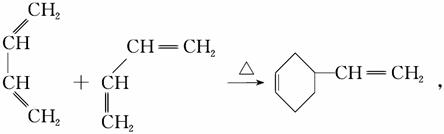 A�Ľṹ��ʽΪ
A�Ľṹ��ʽΪ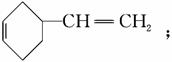 �ٽ����Ϣii��������M��N�Ľṹ�����ɷ�Ӧ������֪BΪ
�ٽ����Ϣii��������M��N�Ľṹ�����ɷ�Ӧ������֪BΪ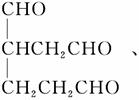 CΪHCHO����1 mol B����3 mol H2�����ӳɷ�Ӧ����M��H2����������Ϊ3 mol��2 g��mol��1��6 g��(5)��(4)��֪CΪHCHO�����N�ķ���ʽC13H8Cl4O2��֪����Ӧ��Ļ�ѧ����ʽΪ
CΪHCHO����1 mol B����3 mol H2�����ӳɷ�Ӧ����M��H2����������Ϊ3 mol��2 g��mol��1��6 g��(5)��(4)��֪CΪHCHO�����N�ķ���ʽC13H8Cl4O2��֪����Ӧ��Ļ�ѧ����ʽΪ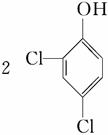 ��HCHO
��HCHO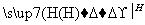
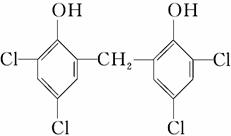 ��H2O��
��H2O��
(6)��(4)��֪A�ķ���ʽΪC8H12�������Ϣii��B��C�Ľṹ��ʽ��֪������������A��ͬ���칹����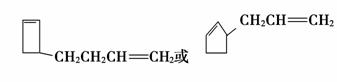 ��
��
�𰸡�(1)1,3������ϩ��(2)a��(3)b
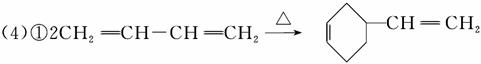
��6
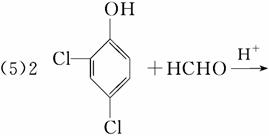
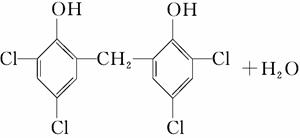
(6)



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1 mL 0.1 mol��L��1��H2SO4��ˮϡ���Ƴ�2 L��Һ���ڴ���Һ����ˮ���������H����Ũ�Ƚӽ���(����)
A��1��10��4 mol��L��1 B��1��10��8 mol��L��1
C��1��10��11 mol��L��1 D��1��10��10 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪���ܵ������ˮ��Һ�д����ܽ�ƽ�⣺
MmAn(s)
 mMn��(aq)��nAm��(aq)
mMn��(aq)��nAm��(aq)
Ksp��[Mn��]m��[Am��]n����Ϊ�ܶȻ���
ijѧϰС����̽��CaSO4����ת��ΪCaCO3�����Ŀ����ԣ�����������ϣ�(25��)
| ���ܵ� ���� | CaCO3 | CaSO4 | MgCO3 | Mg(OH)2 |
| Ksp | 2.8��10��9 mol��2��L��2 | 9.1��10��6 mol��2��L��2 | 6.8��10��6 mol��2��L��2 | 1.8��10��12 mol��3��L��3 |
ʵ�鲽�����£�
����100 mL 0.1 mol��L��1��CaCl2��Һ�м���100 mL 0.1 mol��L��1��Na2SO4��Һ�������а�ɫ�������ɡ�
������������Һ�м������Na2CO3 3 g�����裬���ã���������ȥ�ϲ���Һ��
���ټ�������ˮ���裬���ã�����������ȥ�ϲ���Һ��
��________________________________________________________________________��
(1)��������Ϣ֪KspԽ��ʾ����ʵ��ܽ��Խ______(���С��)��
(2)д���ڢڲ�������Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________
________________________________________________________________________��
(3)��Ƶڢ۲���Ŀ����
________________________________________________________________________��
��4���벹��ڢܲ�����������������
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڻ�̬�����ԭ���У����ں����������������������� (����)��
A������ʧȥ�ĵ����������
B����������С�ĵ����������
C��p�����������һ������s�����������
D�����������������˶��ĵ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ʽṹ�������ʣ������о����ʵ��۽ṹ���������������ʱ仯�ı��ʡ���ش��������⣺
(1)C��Si��N�ĵ縺���ɴ�С��˳����________��C��N��O��F�ĵ�һ�������ɴ�С��˳����________��
(2)A��B��Ϊ�����ڽ���Ԫ�ء����ݱ��е����ݣ�д��Bԭ�ӵĵ����Ų�ʽ��________��
| ������/(kJ��mol��1) | I1 | I2 | I3 | I4 |
| A | 932 | 1 821 | 15 390 | 21 771 |
| B | 738 | 1 451 | 7 733 | 10 540 |
(3)���ɽ���������ˮ�����γɵ�������Ƿ�����ɫ������d��������Ų��йء�һ����ԣ�Ϊd0��d10�Ų�ʱ������ɫ��Ϊd1��d9�Ų�ʱ������ɫ����[Co(H2O)6]2���Էۺ�ɫ���ݴ��жϣ�[Mn(H2O)6]2��________��ɫ(��ޡ����С�)��
(4)L��ԭ�Ӻ������ռ��9�������������һ��δ�ɶԵ��ӣ�L��________(��Ԫ�ط���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ˮ�ȷ��Ʊ�Fe3O4�������ķ�ӦΪ3Fe2����2S2O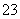 ��O2��xOH��===Fe3O4��S4O
��O2��xOH��===Fe3O4��S4O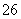 ��2H2O������˵���д������ (����)��
��2H2O������˵���д������ (����)��
A��ÿ����1 mol Fe3O4����Ӧת�Ƶĵ�������Ϊ4 mol
B��Fe2����S2O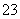 ���ǻ�ԭ��
���ǻ�ԭ��
C��1 mol Fe2��������ʱ����Fe2����ԭ��O2�����ʵ���Ϊ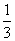 mol
mol
D��x��4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��(Sb)����Ȼ��һ�����������ʽ���ڡ�����������ȡ������һ�������ڸ����½�����ת��Ϊ���������̼��ԭ��
2Sb2S3��3O2��6Fe===Sb4O6��6FeS����
Sb4O6��6C===4Sb��6CO����
���ڷ�Ӧ�١���Ӧ�ڵ�˵����ȷ���� (����)��
A����Ӧ�٢��е��������ֱ���Sb2S3��Sb4O6
B����Ӧ����ÿ����3 mol FeSʱ����ת��6 mol����
C����Ӧ��˵��������Sb�Ļ�ԭ�Ա�Cǿ
D��ÿ����1 mol Sbʱ����Ӧ���뷴Ӧ���������������ʵ���֮��Ϊ1��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ���� �� ��
A��CO2��CH4������������
B��NO2��SO2��������������
C�����ͺͻ����͵���Ҫ�ɷݶ�����֬
D��ë���Ͳ�˿����Ҫ�ɷݶ��ǵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����A-D���������У�������������������������һ�������¾��ܷ�����Ӧ����
| �� | �� | �� | |
| A | Al2O3 | HNO3 | Ba(OH)2 |
| B | NH3 | O2 | H2SO4 |
| C | SiO2 | NaOH | HF |
| D | CO2 | Na2O2 | H2O |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com