
�����谷����ѧʽ��C3N6H6����һ�ַ�ʳƷ�����Ҫ�л�����ԭ�ϣ��㷺�������ϡ���ֽ�����ĵ���ҵ����ͼ���ҹ��Ƽ���������2004�����Ƶ�������Ϊԭ�����������谷�Ĺ��ա�������ѹ����һ�������������¼�������
��֪�������ص��۵���132.7�棬��ѹ�³���160�漴�ɷֽ⣻
�������谷���۵���354�棬����������������ˮ��
��������Ϊԭ�����������谷��ԭ���ǣ�
6CO(NH2)2C3N6H6 + 6NH3+ 3CO2
��ش�
��1��������һ�ֳ��ú�������ߵĻ��ʣ��䵪Ԫ�ص������ٷ���Ϊ ����ʵ����ʹ���ۻ����������ƽ�_________ ����ҵ�Ϻϳ����صĻ�ѧ��Ӧ����ʽΪ___________________________________(��Ӧ�������Բ�д)
��2��д������Ҫ�ɷݵĻ�ѧʽ����Ʒ1 ����Ʒ2 ��X ��
��3������ϵͳ�������з����Ļ�ѧ��Ӧ����ʽΪ�� ��
��4����������������4%��������ģ�ÿ�����ؿ����������谷 �֣�����Ʒ���� �֡�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
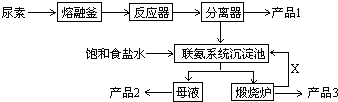 ��2012?�Ϻ�ģ�⣩�����谷����ѧʽ��C3N6H6����һ�ַ�ʳƷ�����Ҫ�л�����ԭ�ϣ��㷺�������ϡ���ֽ�����ĵ���ҵ����ͼ���ҹ��Ƽ����������Ƶ�������Ϊԭ�����������谷�Ĺ���--����ѹ����һ�������������¼�������
��2012?�Ϻ�ģ�⣩�����谷����ѧʽ��C3N6H6����һ�ַ�ʳƷ�����Ҫ�л�����ԭ�ϣ��㷺�������ϡ���ֽ�����ĵ���ҵ����ͼ���ҹ��Ƽ����������Ƶ�������Ϊԭ�����������谷�Ĺ���--����ѹ����һ�������������¼�������| һ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������谷��Ħ������Ϊ126 g/mol | B�������谷��C��N��H��ԭ�Ӹ�����Ϊ1��2��2 | C�������谷��C��N����Ԫ�ص�������Ϊ6��7 | D��1mol�����谷�е�Ԫ�ص�����Ϊ84 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����谷����ѧʽ��C3N6H6����һ�ַ�ʳƷ�����Ҫ�л�����ԭ�ϣ��㷺�������ϡ���ֽ�����ĵ���ҵ����ͼ���ҹ��Ƽ����������Ƶ�������Ϊԭ�����������谷�Ĺ��ա�������ѹ����һ�������������¼�������
��֪�������ص��۵���132��7�棬��ѹ�³���160�漴�ɷֽ⣻
�������谷���۵���354�棬����������������ˮ��
��������Ϊԭ�����������谷��ԭ���ǣ�6 CO��NH2��2 C3N6H6+6 NH3 + 3 CO2
��ش�
1��������һ�ֳ��ú�������ߵĻ��ʣ��䵪Ԫ�ص������ٷ���Ϊ ����ʵ����ʹ���ۻ����������ƽ�_________ ��
2����ҵ�Ϻϳ����صĻ�ѧ��Ӧ����ʽΪ___________________________����Ӧ�������Բ�д��
3��д������Ҫ�ɷݵĻ�ѧʽ����Ʒ1 ����Ʒ2 ��X
4������ϵͳ�������з����Ļ�ѧ��Ӧ����ʽΪ�� ��
5��Ϊ��ʹĸҺ����������IJ�Ʒ2 �����õķ�����
A���������NaCl B���������NaHCO3 C��ͨ��CO2���� D��ͨ��NH3
6����������������4%��������ģ�ÿ�����ؿ����������谷 �֣�
����Ʒ���� �֡�����ȷ��0��001��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�������ʡ�߶���ѧ����ĩ���Ի�ѧ���� ���ͣ������
2008��11��1 ������lOʱ��������֧·12���ൺ��ͨ��ʵҵ����˾�ȵ糧Ժ�ڣ�8 231��25���ﺬ�������谷(�ṹ��ʽΪ��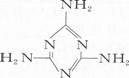 )������Ʒ�ڹ��̡��ʼ�Ȳ��ŵļ���£���ú̿������һ����¯�������٣�
)������Ʒ�ڹ��̡��ʼ�Ȳ��ŵļ���£���ú̿������һ����¯�������٣�
����������Ϣ���ش��������⣺
��1�������谷���� ��
A����� B���߷��ӻ����� C���л��� D��������
��2���������ڴ�������� ��
A��ú̿ B�������谷 C�����������谷���̷� D������ϩ
��3���̷��к��е����ʣ�����ˮ���γɵķ�ɢϵ��Ϊ ������һ��������÷�ɢϵ���ᷢ�� ��
��4�������谷�Ļ�ѧʽ�� �����е�Ԫ�ص���������Ϊ ���������˼���ҵ���̷ۼ�ţ���в��������谷��Ŀ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com