
�Ų��������Ļ�ѧ��������������Fe2O3�������ǵ��ӡ����Ź�ҵ�Ĵ��Բ��ϣ���ҵ�ϲ��������Ѱ۵��½��ϣ�������FeSO4��Һ���Ʊ��Ų��������IJ������£�
�����½��ϣ�������FeSO4��Һ���м�������2 mo1��L-1��H2SO4����Ƥ��
�������������Һ�м���������ˮ�������˳�ȥ���������ʵ���������
�۽������õ���ҺŨ���ᾧ�õ�����A��
�ܽ�����A����ˮ��������NH4HCO3������CO2����ͬʱ�õ�FeCO3��������ɫ��ҺC��
�ݽ�FeCO3����ϴ�ӡ���ɲ����ա��������еı仯Ϊ��FeCO3="FeO+CO2��; " 4FeO+O2="2" Fe2O3��
��������Ϣ�ش��������⣺
��1����18.4mo1��L-1��H2SO4����500mL 2 mo1��L-1H2SO4�����貣��������
mL��Ͳ�����������ձ���500mI������ƿ�⣬����Ҫ ��
��2���������2 mo1��L-1H2SO4����Ƥ�����÷ֱ�Ϊ ��
��3������A�Ļ�ѧʽΪ ��������ҺC�����������ӵķ����� ��
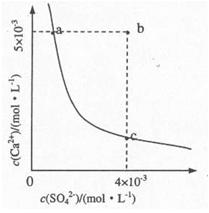
��4������ҺC�м���CaCl2��Һ�ܵõ�CaSO4������������KSP��CaSO4��=9x10-6��������CaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ��
��a���Ӧ��KSP c���Ӧ��KSP����ڡ�����С�ڡ����ڡ�����
������b��䵽a�����д�ʩ���е��� ��
| A����������CaCl2 | B����������BaCl2�� |
| C����������Na2SO4 | D������ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ų��������Ļ�ѧ��������������Fe2O3�������ǵ��ӡ����Ź�ҵ�Ĵ��Բ��ϣ���ҵ�ϲ��������Ѱ۵��½��ϣ�������FeSO4��Һ���Ʊ��Ų��������IJ������£�
�����½��ϣ�������FeSO4��Һ���м�������2 mo1��L-1��H2SO4����Ƥ��
�������������Һ�м���������ˮ�������˳�ȥ���������ʵ���������
�۽������õ���ҺŨ���ᾧ�õ�����A��
�ܽ�����A����ˮ��������NH4HCO3������CO2����ͬʱ�õ�FeCO3��������ɫ��ҺC��
�ݽ�FeCO3����ϴ�ӡ���ɲ����ա��������еı仯Ϊ��FeCO3=FeO+CO2��; 4FeO+O2=2 Fe2O3��
��������Ϣ�ش��������⣺
��1����18.4mo1��L-1��H2SO4����500mL 2 mo1��L-1H2SO4�����貣��������
mL��Ͳ�����������ձ���500mI������ƿ�⣬����Ҫ ��
��2���������2 mo1��L-1H2SO4����Ƥ�����÷ֱ�Ϊ ��
��3������A�Ļ�ѧʽΪ ��������ҺC�����������ӵķ����� ��
��4������ҺC�м���CaCl2��Һ�ܵõ�CaSO4������������KSP��CaSO4��=9x10-6��������CaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ��
��a���Ӧ��KSP c���Ӧ��KSP����ڡ�����С�ڡ����ڡ�����
������b��䵽a�����д�ʩ���е��� ��
A����������CaCl2 B����������BaCl2��
C����������Na2SO4 D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ų��������Ļ�ѧ��������������Fe2O3�������ǵ��ӡ����Ź�ҵ�Ĵ��Բ��ϣ���ҵ�ϲ��������Ѱ۵��½��ϣ�������FeSO4��Һ���Ʊ��Ų��������IJ������£�
�����½��ϣ�������FeSO4��Һ���м�������2 mo1��L-1��H2SO4����Ƥ��
�������������Һ�м���������ˮ�������˳�ȥ���������ʵ���������
�۽������õ���ҺŨ���ᾧ�õ�����A��
�ܽ�����A����ˮ��������NH4HCO3������CO2����ͬʱ�õ�FeCO3��������ɫ��ҺC��
�ݽ�FeCO3����ϴ�ӡ���ɲ����ա��������еı仯Ϊ��FeCO3=FeO+CO2��; 4FeO+O2=2 Fe2O3��
��������Ϣ�ش��������⣺
��1����18.4mo1��L-1��H2SO4����500mL 2 mo1��L-1H2SO4�����貣��������
mL��Ͳ�����������ձ���500mI������ƿ�⣬����Ҫ ��
��2���������2mo1��L-1H2SO4����Ƥ�����÷ֱ�Ϊ ��
��3������A�Ļ�ѧʽΪ ��������ҺC�����������ӵķ����� ��
��4������ҺC�м���CaCl2��Һ�ܵõ�CaSO4������������KSP��CaSO4��=9x10-6��������CaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ��
��a���Ӧ��KSP c���Ӧ��KSP����ڡ�����С�ڡ����ڡ�����
������b��䵽a�����д�ʩ���е��� ��
A����������CaCl2 B����������BaCl2��
C����������Na2SO4 D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣��Ų��������Ļ�ѧ��������������Fe2O3�������ǵ��ӡ����Ź�ҵ�Ĵ��Բ��ϣ���ҵ�ϲ��������Ѱ۵��½��ϣ�������FeSO4�ķ�Һ��Ϊԭ�����Ʊ��Ų���������
��֪�����еĻ�ѧ��Ӧ����ʽΪ��FeCO3 �� FeO + CO2����4FeO + O2 �� 2Fe2O3
��1����98%��H2SO4������500mL��20%��H2SO4�����貣��������
A�������� B���ձ� C��©�� D��250mL����ƿ
E��500mL����ƿ F����ͷ�ι�
��2��Ũ���ᾧ��õ��ľ����� ���ѧʽ����A�������� ��
����Һ�и����ӵ�Ũ�ȱȽϴ�СΪ�� ��
��3��20%H2SO4����Ƥ�����÷ֱ��� ��
��4��������Һ�к���NH4+�ķ�����
��5��д�����衰�ϳɡ��з����Ļ�ѧ�仯���û�ѧ����ʽ��ʾ����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��㶫ʡ��У������ѧ�ڵڶ������������ۣ���ѧ���� ���ͣ�ʵ����
��16�֣��Ų��������Ļ�ѧ��������������Fe2O3�������ǵ��ӡ����Ź�ҵ�Ĵ��Բ��ϣ���ҵ�ϲ��������Ѱ۵��½��ϣ�������FeSO4�ķ�Һ��Ϊԭ�����Ʊ��Ų���������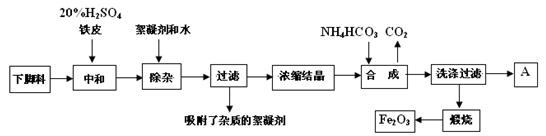

��֪�����еĻ�ѧ��Ӧ����ʽΪ��FeCO3 �� FeO + CO2����4FeO + O2 �� 2Fe2O3
��1����98%��H2SO4������500mL��20%��H2SO4�����貣��������
| A�������� | B���ձ� | C��©�� | D��250mL����ƿ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com