
���ⶨ�������Ȼ��Ƶ�С�մ��̬��Ʒ��NaHCO3�����������ɲ����������ַ�����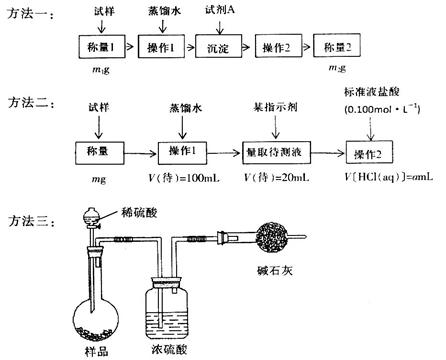
�����ģ���ʹ�û�ѧ�Լ���ʹ��ʵ���ҳ���������
��Ҫ��ش��������⣺
��1������һ�ǽ�HCO3-����ת��Ϊ���������أ����Լ�AΪ______________���ѧʽ����Һ������2����_______________________��
��2������������1��Ҫ�õ��IJ����������ձ���������������Ҫ___________________������2��������__________������Ʒ��NaHCO3����������Ϊ_________���ú�m��a�ı���ʽ��ʾ����
��3�����ݷ����������õ�ʵ��װ�ã����˳������������⣬����ⶨ��ʵ��������_______________����ϸ������ʵ��װ�ã��ɴ˲�õ����ݼ������ʵ�����п���ƫ��Ҳ�п���ƫ�ͣ�ƫ�ߵ�ԭ�������______________________________��ƫ�͵�ԭ�������____________________________��
��4�������ĵ�ʵ��ԭ����________________���û�ѧ����ʽ��ʾ����
��9�֣���1��Ba(OH)2[��Ca(OH)2]�����ˡ�ϴ�ӡ�����д����÷֣�
��2��100ml����ƿ����ͷ�ιܣ��к͵ζ���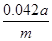 ����
����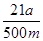 ��
��
��3��m(CO2)����ʵ��ǰ�����ܵ��������������еĶ�����̼��ˮ�����Ƚ������ܣ�װ���еĶ�����̼û����ȫ��������
��4��2NaHCO3 Na2CO3��H2O��CO2����ÿ��1�֣�
Na2CO3��H2O��CO2����ÿ��1�֣�
���������������1��Ҫ��HCO3-����ת��Ϊ���������أ�����Ҫ����ǿ�������������������ƣ���ѧʽ�ֱ�ΪBa(OH)2��Ca(OH)2�����������ʴ���Һ�з�����IJ����ǹ��ˣ����õij�������Ҫ����ϴ�Ӳ��������ܳ�����
��2��Ҫ����100ml����Һ�������1��Ҫ�õ��IJ��������������ձ����������⣬����Ҫ100ml����ƿ���Լ�����ʱ�Ľ�ͷ�ιܡ�������ζ�̼��������Һ�����Բ���2���������к͵ζ���20ml����Һ������������ʵ�����a��10��4mol������ݷ���ʽNaHCO3��HCl��NaCl��H2O��CO2����֪��ԭ��Ʒ��̼�����Ƶ����ʵ�����a��10��4mol��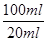 ��5a��10��4mol����������5a��10��4mol��84g/mol��0.042ag�����Ը���Ʒ��NaHCO3����������Ϊ
��5a��10��4mol����������5a��10��4mol��84g/mol��0.042ag�����Ը���Ʒ��NaHCO3����������Ϊ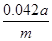 ��
��
��3��̼�����������ᷴӦ����CO2����ͨ������CO2���������������̼�����Ƶĺ��������Ը��ݷ����������õ�ʵ��װ�ã����˳������������⣬����ⶨ��ʵ��������m(CO2)��ʵ��ǰ�����ܵ����������ڸ������������������Կ����еĶ�����̼��ˮ�����Ƚ������ܣ����²������ƫ�ߣ�����װ���еĶ�����̼û����ȫ�������ܣ����в��࣬��˻ᵼ�²������ƫ�͡�
��4������̼�����Ʋ��ȶ��������ֽ�����̼���ơ�ˮ��CO2���ݴ�Ҳ���Բ���̼�����Ƶĺ��������Է����ĵ�ʵ��ԭ����2NaHCO3 Na2CO3��H2O��CO2����
Na2CO3��H2O��CO2����
���㣺�����Ȼ�����Ʒ��̼�����ƺ����ⶨ��ʵ�鷽�����������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС���������ռ�����Ϣ���ơ�þ�Ȼ��ý���������CO2������ȼ�ա����Ƕ�����CO2������ȼ�պ�õ��İ�ɫ�������������̽����
��ʵ���������ȼ�յ���Ѹ������װ��CO2�ļ���ƿ�У��������м���ȼ�գ���Ӧ����ȴ��ƿ���к�ɫ������ƿ���ϸ��Ű�ɫ���ʡ�
��������衿
����1����ɫ������Na2O��
����2����ɫ������Na2CO3��
����3����ɫ������Na2O��Na2CO3�Ļ���
����Ʒ�������С���ȼ�պ����ɵİ�ɫ���ʽ�������̽����
| ʵ�鷽�� | ʵ����� | ʵ������ | ���� |
| ����1 | ȡ������ɫ�������Թ��У���������ˮ������Ʒȫ������ˮ�������м�����ɫ��̪��Һ | ��Һ��ɺ�ɫ | ��ɫ����ΪNa2O |
| ����2 | ��ȡ������ɫ�������Թ��У���������ˮ������Ʒȫ������ˮ�������м��������CaCl2��Һ���ھ���Ƭ�̣�ȡ�ϲ���Һ���Թ��У��μ���ɫ��̪��Һ | �ٳ��ְ�ɫ���� ������������ | ��ɫ����ΪNa2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
һ��Ũ��NaHCO3��Һ��CuSO4��Һ��Ӧ������������ɫ����״�������ͳ����ɷ�������������ּ��裺
����һ��������CuCO3���������������Cu��OH��2��
��������������CuCO3��Cu��OH��2�Ļ���
��1��д���������������Cu��OH��2���ɵ����� �������ӷ���ʽ��ʾ����
��2��Ϊ��̽�������ijɷ֣�ȡ����һ���ֳ������μ�ϡ���ᣬ������ų���ƾ�������жϳ����к��� _��
��3��Ϊ�˽�һ��̽�������ijɷ֣�����ȷ�������к��ּ�����������ʵ�飬װ��ͼ���£�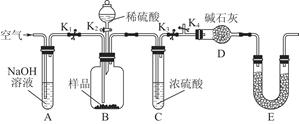
���о����������ǰ���뽫��������Һ�з��벢�����������������Ϊ _ ��ϴ�ӡ����
��װ��E��ҩƷ�������� __����Ϊ __��
��ʵ������������²������裺a.��K1��K3���ر�K2��K4��ͨ������������˲���������� ��
b���ر�K1��K3����K2��K4����ַ�Ӧ��c.��ͨ���������ʱ���������ڴ��� _���رյ��� _��
����������Ʒ������Ϊm g��װ��D������������n g����������ƷΪ�����m�� n֮��Ĺ�ϵΪ _
����������������Cu��OH��2����������Ϊ _���������в���c�����ʹ��ý�� ���ƫ�ߡ�����Ӱ�족��ƫ�͡�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij����С���������ͼ��ʾ��ʵ��װ�ý���ʵ�飬�ش��������⣺
��1����ʼʵ��ʱ����e�Ǵģ���ƿA�з�����Ӧ�����ӷ���ʽΪ��______________���Լ�ƿB�п��Թ۲쵽��������_____________________��
��2�������Ӻ���ϼ���e�����Լ�ƿB�п��Թ۲쵽��������____��B�з�Ӧ�����ӷ���ʽ��_____________��
��3�������������f�ŵ�Լ2/3Һ�壬��ʱ�Լ�ƿB�п��ܳ��ֵ�������____________________��B�з�Ӧ�Ļ�ѧ����ʽ��_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijС���ͬѧ�Է����ѾõĹ������Ƶijɷֽ���̽������ش��������⣺
(1)��Ӧ2Na2O2��2H2O===4NaOH��O2���Ļ�ԭ����________(д��ѧʽ)����Ӧ2Na2O2��2CO2===2Na2CO3��O2�У�ÿ����1 mol O2ת��________mol���ӡ�
(2)����̽����
��������衣
����1��ȫ����Na2O2
����2��ȫ����Na2CO3
����3��________________
����Ʒ���������ʵ�顣��д��ʵ�鲽���Լ�Ԥ������ͽ���(�ɲ�����)��
��ѡʵ���Լ�������������ˮ��1 mol��L��1 H2SO4��Һ������ʯ��ˮ������-KI��Һ����̪��Һ������Թܡ�С�ձ�����ͷ�ιܡ�
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����������Ʒ���Թ��У��μ�����1 mol��L��1 H2SO4��Һ��Ȼ���ڱڸ��г���ʯ��ˮ���ձ������Թܿ� | ������ʯ��ˮδ�����ǣ������1������ ������ʯ��ˮ����ǣ���______________________ |
| ����2�� | |
| ����3�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���������䡢��ˮ��Ӧ����������ˮ������Ӧ��ʵ��װ����ͼ��ʾ����Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ���������ˮ������Ӧ��ʵ�顣��ش�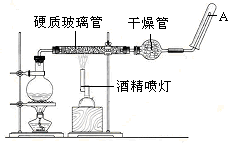
��1��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��2��ʵ��ǰ���������װ�ý��еIJ�����________��ʵ�鿪ʼʱӦ�ȵ�ȼ ����ƾ��ơ��ƾ���ơ�����ʵ�����ʱӦ��Ϩ�� ����ƾ��ơ��ƾ���ơ�����
��3��Բ����ƿ��ʢ��ˮ����װ�����Ⱥ����Ҫ������ ����ƿ��Ӧ���ȷ��� ���������� ���������ʢװ��������________��
��4����Ӳ�ʲ�������ȴ��ȡ�������еĹ�����������ϡ�������ҺB��ȡ������ҺB�μ� ��Һ���� ����ʵ��������˵����ҺB�к���Fe3+��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ�˼����г��ϳ������ֽ�������ƿ����Ҫ�ɷ֣�ijС��ȡ�ס�����������ƿ�����������Ϊ��Ʒ���ֱ������������ʵ�飺
I��ȡ������Ʒ���Թ��У��������ᣬ��ַ�Ӧ����ˡ�
II��ȡ������Һ���Թ��У������еμ�����������Һ��
����II��ʵ���������£�
| ��Ʒ�� | ���ɰ�ɫ�����������μ�����������Һ��������ʧ |
| ��Ʒ�� | ���ɰ�ɫ��������ɫ����Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����������������̼��Ӧ���ڡ����ߡ��ĺ�����С����Ա̫������ʱ���ĺ�����С���DZͧ�о�ʹ�ù��������������������˺���֮�á�ijѧ��ѡ�ô���ʯ�������һ������������ҩƷ���ʵ�飬��֤��һ��ʵ��
���������װ�ûش��������⣺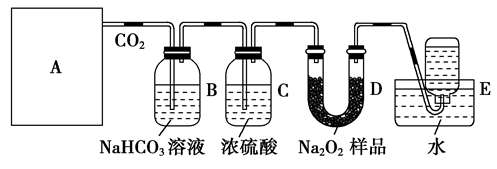
��1��A����ȡCO2��װ�ã����ѡ������װ���е�________(�����)��
��2��Bװ���з��������ӷ���ʽ��_____________________________________________��
Cװ�õ�������_____________________________________________________��
��3��Dװ���з�����Ӧ�Ļ�ѧ����ʽ��______________________________________��
��4��Ҫ��õ��ϴ���������������װ��D��E֮������һʢ��__ ______(��д�Լ�����)��________(��дװ������)��
��5����μ��鼯��ƿ���ռ���������_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧС��ѧ��������ͼ��ʾװ�ý��С�����ˮ��Ӧ����ʵ�飬��̽���������ɷ�(ͼ�мгּ�β������װ�þ�����ȥ)��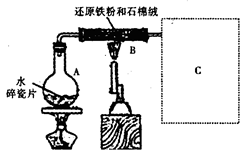
��1��װ��B�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2�����߿�ͼC�������ռ�װ������ͼ�е� ��ѡ����ĸ��ţ���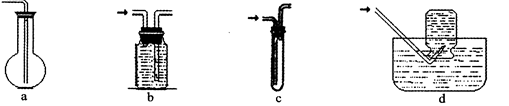
��3����Ӧֹͣ����B����ȴ�������õ���������Ϊ28��8g����������м������ϡ�����ַ�Ӧ������������ʵ�飺 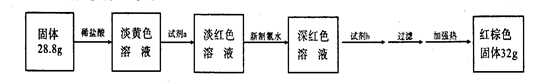
�Լ�a�Ļ�ѧʽ�� ���Լ�b�Ļ�ѧʽ�� ������������ˮ����Һ��ɫ�����ԭ����(�����ӷ���ʽ��ʾ) ��
��4��ijͬѧ��ʵ���м����˹�����ˮ������һ��ʱ��������ɫ��ȥ���������ɫԭ�����̽��������������衣����1����Һ�е�+3����������Ϊ���ߵļ�̬������2��
��Ϊ�˶�������ļ���2������֤��ʵ�鷽����
��
��5�������������ݣ��ɼ������Ӧ��Bװ������Ԫ�ص���������Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com