
����Ŀ��ijͬѧ��Ҫ����450 mL 0.5 mol��L��1��NaOH��Һ����ش��������⣺
��1����ʵ��������õ��IJ��������У�����������Ͳ���ձ���______________����ͷ�ιܡ��Լ�ƿ��
��2����������ƽ����ʱ��Ӧ��NaOH����________��������ȡ�Ĺ�������Ϊ_______��
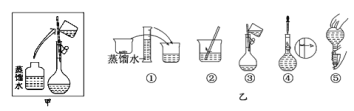
��3������ʱ������������ͼ��ʾ�����ͼ����Ӧ����ͼ�е�___(��ѡ����ĸ)֮�䡣
A������ڡ� B������ۡ� C������ܡ� D�������
��4�����ƹ�����ϴ���ձ���������2��3�ε�Ŀ����______________________��
��5�����ݵμ�����ˮʱ�������������˿̶��ߣ������ķ�����______________��
��6����ͬѧʵ������NaOH��Һ��Ũ��Ϊ0.6 mol��L��1��ԭ�������____(�����)��
a��������������
b��ϴ��������ƿ�в���������ˮ
c������NaOH����ʱ�������ˡ��������
d������ʱ���ӿ̶���
e������ҡ�Ⱥ���Һ����ڿ̶����ּ�������ˮ�����̶���
f���ܽ������ձ���Һ��δϴ��
g������ǰ��Һδ������ȴ
���𰸡�500 mL����ƿ С�ձ� 10.0g C ��֤����ȫ��ת��������ƿ�� �������� a d g
��������
(1)��������һ�����ʵ���Ũ����Һ��һ�㲽�裬ѡ����Ҫ��������
(2)����450mL��Һ����Ҫѡ��500mL����ƿ������n=cV��m=nM�������Ҫ�������������ƹ����������
(3)��ͼ��ʾ�IJ���Ϊ��Һϴ�Ӻ�������ƿ�ڼ�ˮ��������Ʋ����ж���
(4)ת����Һʱ��Ҫ�Ѳ������ձ��е�����ȫ��ת������ƿ��
(5)������Һʱ���ִ��������Ҫ�������ƣ�
(6)�����������������ʵ����ʵ�������Һ�����Ӱ�죬����c=![]() ������������
������������
(1)����һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢����(��ȡ)���ܽ�(ϡ��)����ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ��õ���������������ƽ��ҩ�ס���Ͳ���ձ�����������500mL����ƿ����ͷ�ιܣ����Ի�ȱ�ٵ�������500mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��500mL����ƿ��
(2)ʵ����û��450mL������ƿ������ʱ��Ҫѡ��500mL����ƿ��ʵ�������Ƶ���450mL0.5 mol/LNaOH��Һ����Ҫ�������ƹ���õ�����Ϊ��m=40g/mol��0.5 mol/L��0.5L=10.0g���������ƾ���ǿ��ʴ�ԣ������׳��⣬����ʱӦ�÷����ձ��п��ٳ������ʴ�Ϊ���ձ���10.0g��
(3)��ͼ��ʾ�IJ���Ϊ��Һϴ�Ӻ�������ƿ�ڼ�ˮ��Ӧ��ת���붨��֮�䣬��Ӧ�ڢۺ͢�֮�䣬��ѡ��C��
(4)���ձ��е���Һת�Ƶ�����ƿʱ���ձ��л��в������������ƣ�Ϊ�˱�֤����ȫ��ת��������ƿ�У�Ҫϴ���ձ�2��3�Σ�����ϴҺת������ƿ���ʴ�Ϊ����֤����ȫ��ת��������ƿ�У�
(5)�ڶ��ݵμ�����ˮʱ�������������˿̶��ߣ���Һ�����ƫ��Ũ��ƫС��Ҫ�������ƣ��ʴ�Ϊ���������ƣ�
(6)��ͬѧʵ������NaOH��Һ��Ũ��Ϊ0.6molL-1��Ũ��ƫ�ߡ�a��������մ�����ʣ���ȡ���ʵ�����ƫ�����ʵ����ʵ���ƫ����Һ��Ũ��ƫ��aѡ��b������ƿ��ԭ����������ˮ�������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣬��b��ѡ��c������NaOH����ʱ������������������������Ϊʵ�������û���õ����룬���Զ����ʵ����ʵ���������Ӱ�죬��ҺŨ�Ȳ��䣬��c��ѡ�� d������ʱ�����ӿ̶��ߣ�������Һ�����ƫС����Һ��Ũ��ƫ��dѡ��e������ҡ�Ⱥ���Һ����ڿ̶����ּ�������ˮ�����̶��ߣ�������Һ���ƫ��Ũ��ƫС����e��ѡ��f���ܽ������ձ���Һ��δϴ�ӣ����²������������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���f��ѡ��g��ת����Һʱδ����ȴ����ȴ��Һ���½�����Һ���ƫС����ҺŨ��ƫ�ߣ���gѡ���ʴ�Ϊ��adg��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ӧ2SO2(g) + O2(g) ![]() 2SO3(g) ��H = a kJ/mol�������仯��ͼ��ʾ������˵����������ȷ���ǣ�������
2SO3(g) ��H = a kJ/mol�������仯��ͼ��ʾ������˵����������ȷ���ǣ�������
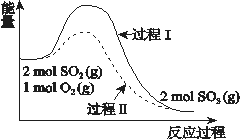
A. 2SO2(g) + O2(g) ![]() 2SO3(l) ��H > a kJ/mol
2SO3(l) ��H > a kJ/mol
B. ����II����ʹ���˴�����ʹ�ô������������SO2��ƽ��ת����
C. ��Ӧ��ϼ���������֮��С��������ɼ��ͷ�����֮��
D. ��2molSO2(g) ��1mol O2(g)����һ�ܱ������г�ַ�Ӧ��ų������յ�����С����a�� kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������CO2������ɳ�����չ����Ҫս��֮һ��
��1����̫���ܹ��ղ���CO2�ɵ�̿�ڣ���������ͼ��ʾ:

�ٲ���1mo1CO2ת�Ƶ��ӵ����ʵ�����_________��
�ڹ���2��Ӧ�Ļ�ѧ����ʽ��________��
��2����CO2������ɺϳɵ�̼ϩ����2CO2(g)��6H2(g)![]() C2H4(g)��4H2O(g)����Ͷ�ϱ�n(CO2):n(H2)=1:3��CO2��H2�����ܱ���������0.1MPaʱ�����ƽ��ʱ������̬���ʣ����¶�(T)�����ʵ���(n)�Ĺ�ϵ��ͼ��ʾ��
C2H4(g)��4H2O(g)����Ͷ�ϱ�n(CO2):n(H2)=1:3��CO2��H2�����ܱ���������0.1MPaʱ�����ƽ��ʱ������̬���ʣ����¶�(T)�����ʵ���(n)�Ĺ�ϵ��ͼ��ʾ��

������Ӧ���ʱ���H_________0��
�����CO2��ת���ʣ��ɲ��õķ�����______��
a.����n(CO2)��n(H2)��Ͷ�ϱ�
b.�ı����
c.���������
��ͼ�б�ʾˮ��������_____��
��3�����CO2���Ƶö���ȼ�ϣ���ͼ�������Ե������Һ�У��Զ��Բ������缫��CO2ת��Ϊ��ϩ��ԭ��ģ�͡�

��̫���ܵ�ص�������________��
�����ɱ�ϩ�ĵ缫��Ӧʽ��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ȡһ��������ò��õķ�����( )
A.������������ӦB.�������Ȼ��ⷴӦ
C.��ϩ��������ӦD.��ϩ���Ȼ��ⷴӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���밴Ҫ�����������գ�
��1������֮��Ϊ16��7��6��SO2��CO��NO���Ӹ���֮��Ϊ______��
��2��1.204��1024��D2O������Ϊ______��
��3��9.2g NOx�к���Nԭ����Ϊ0.2mol����x��ֵΪ______��
��4�� 1L1mol/L��AlCl3��Һ�к�1.5L______mol/L��MgCl2��Һ�е�������Ũ����ȡ�
��5���ڱ�״���£�CO��CO2�Ļ�����干39.2 L������Ϊ61 g����������������ʵ���֮��Ϊ_______mol������CO2Ϊ__________mol��
��6������100mL 2mol/L��ϡHCl��Һ����������м��Ӧ�������ɵ�FeCl2���400mL��Һ������Һ��FeCl2�����ʵ���Ũ����______________��
��7���ڱ�״���£���224L�Ȼ�����������635mL��ˮ���ܶ�Ϊ1g/cm3���У�����������ܶ�Ϊ1.18g/cm3�������������ʵ���������____________������������ʵ���Ũ��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������г��õ�ij������X�Ľṹ��ʽΪ: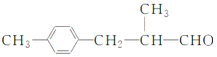
��֪:
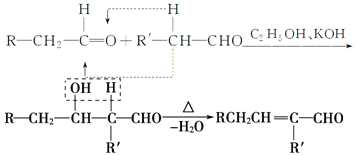
����X�ĺϳ�·������:
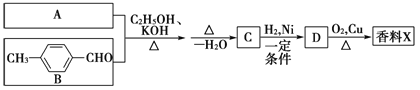
(1)A�Ľṹ��ʽ��________________��
(2)�����л���C�к���̼̼˫�������õ��Լ�_____________��
a��������Һ b�����Ը��������Һ
c����ˮ d������������Һ
(3)D��X�Ļ�ѧ����ʽΪ____________________________��
(4)�л���B��ij��ͬ���칹��E,������������:
a����Ũ��ˮ��Ӧ���ɰ�ɫ����,��1 mol E�������4 mol Br2��Ӧ
b�����������ʾ���л����д���̼̼˫��
��E�Ľṹ��ʽΪ________________��
(5)�����ͪ(![]() )��һ����Ҫ��ҽҩ�м���,��ο������ϳ�·�ߣ����һ���ɱ�ϩ[CH3CH=CH2]�Ϳ�ȩ(
)��һ����Ҫ��ҽҩ�м���,��ο������ϳ�·�ߣ����һ���ɱ�ϩ[CH3CH=CH2]�Ϳ�ȩ(![]() )Ϊԭ���Ʊ������ͪ�ĺϳ�·��(���Լ�����,�ýṹ��ʽ��ʾ�л���),�ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ����)____________________��
)Ϊԭ���Ʊ������ͪ�ĺϳ�·��(���Լ�����,�ýṹ��ʽ��ʾ�л���),�ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ����)____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ������������ԭ��Ӧ�������������ֻ�����Ӧ���͵��� ( )
A. CuO + H2 ![]() Cu + H2O B. Fe2O3 + 3CO
Cu + H2O B. Fe2O3 + 3CO ![]() 2Fe + 2CO2
2Fe + 2CO2
C. 2KMnO4 ![]() K2MnO4 + MnO2 + O2�� D. NaOH + HCl = NaCl + H2O
K2MnO4 + MnO2 + O2�� D. NaOH + HCl = NaCl + H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ֶ���������Ԫ��X��Y��Z��W��ԭ���������������������X��Y��Z����Ԫ����ɣ�25��ʱ��0.01mol/L ����Һ�е�c(OH-)/c(H+)=1010��Z��W ͬ���ڣ���W������ϼ�����ͻ��ϼ۵Ĵ�����Ϊ4������˵������ȷ����
A. �����ʵ����Ļ�����Z2Y2 ��Z2W�������Ӹ�����ͬ
B. ԭ�Ӱ뾶X
C. մ��W�ĵ��ʵ��Թܿ��þƾ�ϴ��
D. ���⻯����ȶ���Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X2+ ��Y- ��벣�18Ar���ĵ��Ӳ�ṹ��ͬ�������ж��в���ȷ����
A.ԭ�Ӱ뾶�� X>YB.ԭ�������� X>Y
C.������������ X>YD.���Ӳ����� X>Y
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com