
(15·Ö)ĮņĖįŃĒĢśļ§ÓÖ³ĘÄŖ¶ūŃĪ£¬ŹĒĒ³ĀĢÉ«¾§Ģ唣ĖüŌŚæÕĘųÖŠ±ČŅ»°ćŃĒĢśŃĪĪČ¶Ø£¬ŹĒ³£ÓƵÄFe2+ŹŌ¼Į”£Ä³ŹµŃ銔×éĄūÓĆ¹¤Ņµ·ĻĢśŠ¼ÖĘČ”ÄŖ¶ūŃĪ£¬²¢²ā¶ØĘä“æ¶Č”£
ŅŃÖŖ:¢Ł
¢ŚÄŖ¶ūŃĪŌŚŅŅ“¼ČܼĮÖŠÄŃČÜ”£
¢ń£®ÄŖ¶ūŃĪµÄÖĘČ”
ŹŌ·ÖĪö£ŗ
£Ø1£©²½Öč2ÖŠ¼ÓČČ·½Ź½ £ØĢī”°Ö±½Ó¼ÓČČ”±©p”°Ė®Ō”¼ÓČČ”±»ņ”°É³Ō””±£©£»±ŲŠėŌŚĢśŠ¼ÉŁĮæŹ£ÓąŹ±£¬½ųŠŠČČ¹żĀĖ£¬ĘäŌŅņŹĒ ”£
£Ø2£©²½Öč3ÖŠ°üŗ¬µÄŹµŃé²Ł×÷Ćū³Ę ”£
£Ø3£©²śĘ·ÄŖ¶ūŃĪ×īŗóÓĆ Ļ“µÓ£ØĢī×ÖÄø±ąŗÅ£©”£
a£®ÕōĮóĖ® b£®ŅŅ“¼ c£®ĀĖŅŗ
¢ņ£®ĪŖ²ā¶ØĮņĖįŃĒĢśļ§(NH4)2SO4?FeSO4?6H2O¾§Ģå“æ¶Č£¬Ä³Ń§ÉśČ”m gĮņĖįŃĒĢśļ§ŃłĘ·ÅäÖĘ³É500 mLČÜŅŗ£¬øł¾ŻĪļÖŹ×é³É£¬¼×”¢ŅŅ”¢±ūČżĪ»Ķ¬Ń§Éč¼ĘĮĖČēĻĀČżøöŹµŃé·½°ø£¬Ēė»Ų“š£ŗ
(¼×)·½°øŅ»£ŗČ”20.00 mLĮņĖįŃĒĢśļ§ČÜŅŗÓĆ0.1000 mol”¤L£1µÄĖįŠŌKMnO4ČÜŅŗ·ÖČż“Ī½ųŠŠµĪ¶Ø”£
(ŅŅ)·½°ø¶ž£ŗČ”20.00 mLĮņĖįŃĒĢśļ§ČÜŅŗ½ųŠŠČēĻĀŹµŃ锣
£Ø1£©ČōŹµŃé²Ł×÷¶¼ÕżČ·£¬µ«·½°øŅ»µÄ²ā¶Ø½į¹ū×ÜŹĒŠ”ÓŚ·½°ø¶ž£¬ĘäæÉÄÜŌŅņĪŖ
£¬ŃéÖ¤ĶĘ²āµÄ·½·ØĪŖ£ŗ ”£
(±ū)·½°øČż£ŗ(ĶعżNH4+²ā¶Ø)ŹµŃéÉč¼ĘĶ¼ČēĻĀĖłŹ¾”£Č”20.00 mLĮņĖįŃĒĢśļ§ČÜŅŗ½ųŠŠøĆŹµŃ锣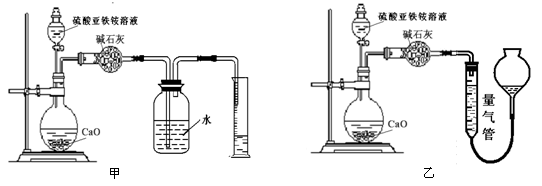
£Ø2£©¢Ł×°ÖĆ £ØĢī”°¼×”±»ņ”°ŅŅ”±£©½ĻĪŖŗĻĄķ£¬ÅŠ¶ĻĄķÓÉŹĒ ”£ĮæĘų¹ÜÖŠ×ī¼ŃŹŌ¼ĮŹĒ £ØĢī×ÖÄø±ąŗÅ”£ČēŃ””°ŅŅ”±ŌņĢī“ĖæÕ£¬ČēŃ””°¼×”±“ĖæÕæɲ»Ģī£©”£
a£®Ė® b£®±„ŗĶNaHCO3ČÜŅŗ c£®CCl4
¢ŚČō²āµĆNH3µÄĢå»żĪŖV L(ŅŃÕŪĖćĪŖ±ź×¼×“æöĻĀ)£¬ŌņøĆĮņĖįŃĒĢśļ§¾§ĢåµÄ“æ¶ČĪŖ ”£
£Ø15·Ö£©
I£®£Ø1£©Ė®Ō”¼ÓČČ(1·Ö )
·ĄÖ¹Fe2+±»Ńõ»Æ£¬Ķ¬Ź±ČČ¹żĀĖæÉ·ĄÖ¹ĮņĖįŃĒĢśŅŌ¾§ĢåŠĪŹ½Īö³ö£Ø2·Ö£©
£Ø2£©¼ÓČČÕō·¢£¬ÅØĖõ½į¾§£Ø2·Ö£©
£Ø3£© b£Ø1·Ö £©
II£®£Ø1£© Fe2+Ņѱ»æÕĘų²æ·ÖŃõ»Æ (2·Ö)
ȔɣĮæĮņĖįŃĒĢśļ§ČÜŅŗ£¬¼ÓČėÉŁĮæKSCNČÜŅŗ£¬ČōČÜŅŗ±äĪŖŃŖŗģÉ«£¬ĖµĆ÷Fe2+ŅŃ
±»æÕĘų²æ·ÖŃõ»Æ (2·Ö)
£Ø2£©¢ŁŅŅ£Ø1·Ö£© ¼××°ÖĆ»į³öĻÖµ¹Īü(1·Ö) c £Ø1·Ö£©
¢Ś (2·Ö)
(2·Ö)
½āĪöŹŌĢā·ÖĪö£ŗI.£Ø1£©²½Öč2ÖŠµÄĪĀ¶ČæŲÖĘŌŚ70-75”ę£¬ĖłŅŌŃ”ŌńĖ®Ō”¼ÓČČ£»Fe2+Ņ×±»ŃõĘųŃõ»Æ£¬¶ųĒŅĪĀ¶Č½µµĶĮņĖįŃĒĢśµÄČܽā¶Č¼õŠ”£¬ĖłŅŌ±ŲŠėŌŚĢśŠ¼ÉŁĮæŹ£ÓąŹ±£¬½ųŠŠČČ¹żĀĖ£¬ĘäŌŅņŹĒ·ĄÖ¹Fe2+Ņ×±»Ńõ»Æ£¬Ķ¬Ź±ČČ¹żĀĖæÉ·ĄÖ¹ĮņĖįŃĒĢśŅŌ¾§ĢåŠĪŹ½Īö³ö£»
£Ø2£©“ÓĮņĖįŃĒĢśļ§ĀĖŅŗµ½¾§Ģ壬֊¼äŠčŅŖ¾¹ż¼ÓČČÕō·¢£¬ÅØĖõ½į¾§£»
£Ø3£©ŅņĪŖÄŖ¶ūŃĪŌŚŅŅ“¼ČܼĮÖŠÄŃČÜ£¬ĖłŅŌŃ”ŌńÓĆŅŅ“¼Ļ“µÓ£¬“š°øŃ”b£»
II.£Ø1£©ÓĆĖįŠŌøßĆĢĖį¼ŲČÜŅŗµĪ¶Ø£¬ŌĄķŹĒŃĒĢśĄė×Ó±»ĖįŠŌøßĆĢĖį¼ŲČÜŅŗŃõ»Æ£¬ĄūÓĆĖłÓĆČÜŅŗµÄĢå»ż¼ĘĖćŃĒĢśĄė×ÓµÄĮ棬“Ó¶ų¼ĘĖć¾§ĢåµÄ“æ¶Č£»ÓĆĀČ»Æ±µČÜŅŗµĪ¶Ø£¬ŌĄķŹĒĮņĖįøłĄė×ÓÓė±µĄė×Ó·“Ӧɜ³ÉĮņĖį±µ³Įµķ£¬ĄūÓĆ³ĮµķµÄÖŹĮæ¼ĘĖćĮņĖįøłĄė×ÓµÄĮ棬“Ó¶ų¼ĘĖć¾§ĢåµÄ“æ¶Č”£ĮņĖįøłĄė×Ó²»»į·¢Éś±ä»Æ£¬ĖłŅŌ·½°øŅ»µÄ²ā¶Ø½į¹ū×ÜŹĒŠ”ÓŚ·½°ø¶ž£¬ĘäæÉÄÜŌŅņĪŖFe2+Ņѱ»æÕĘų²æ·ÖŃõ»Æ£»ŃéÖ¤ĶĘ²āµÄ·½·Ø¼“ŹĒŃéÖ¤ČÜŅŗÖŠŹĒ·ń“ęŌŚĢśĄė×Ó£¬¾ßĢå²Ł×÷ŹĒȔɣĮæĮņĖįŃĒĢśļ§ČÜŅŗ£¬¼ÓČėÉŁĮæKSCNČÜŅŗ£¬ČōČÜŅŗ±äĪŖŃŖŗģÉ«£¬ĖµĆ÷Fe2+Ņѱ»æÕĘų²æ·ÖŃõ»Æ£»
£Ø2£©¢Ł×°ÖĆŅŅ±Č½ĻŗĻĄķ£¬ŅņĪŖ¼××°ÖĆ»į³öĻÖµ¹Īü£¬ĪŽ·Ø²āĮæÉś³ÉĘųĢåµÄÖŹĮæ£»Ń”ŌńŅŅ×°ÖĆ£¬ŌņĮæĘų¹ÜÖŠŹ¢·ÅµÄŹŌ¼ĮŹĒĖÄĀČ»ÆĢ¼£¬ŅņĪŖ°±ĘųŅ×ČÜÓŚĖ®£¬²»ČÜÓŚĖÄĀČ»ÆĢ¼£¬²āĮæŹż¾Ż±Č½Ļ×¼Č·£¬ĖłŅŌ“š°øŃ”c£»
¢Śøł¾ŻĮņĖįŃĒĢśļ§µÄ»ÆѧŹ½µĆ³ö2NH3”«(NH4)2SO4?FeSO4?6H2O£¬ĖłŅŌ500mLČÜŅŗÖŠĮņĖįŃĒĢśļ§¾§ĢåµÄÖŹĮæĪŖVL/22.4L/mol/2”Į392g/mol”Į25£¬Ęä“æ¶ČĪŖVL/22.4L/mol/2”Į392g/mol”Į25/mg”Į100%= ”£
ӣ
æ¼µć£ŗæ¼²éĪļÖŹÖʱø£¬²Ł×÷µÄÅŠ¶Ļ£¬Ąė×ӵļģŃ飬“æ¶ČµÄ¼ĘĖć


 ĶسĒѧµäĬŠ“ÄÜŹÖĻµĮŠ“š°ø
ĶسĒѧµäĬŠ“ÄÜŹÖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ä³Ń§ÉśÓĆŹµŃéŹŅ³£¼ūµÄĖį”¢¼ī”¢ŃĪŗĶ½šŹōµ„ÖŹĪŖ·“Ó¦Īļ£¬²¢ĄūÓĆŅ»øöµ×²æÓŠŠ”æ׵ďŌ¹ÜŗĶŅ»øö¹ćæŚĘæ×é×°³ÉČēĶ¼ĖłŹ¾µÄ×°ÖĆ”£ŹŌ»Ų“š£ŗ
(1)ČōŹŌ¹Ü֊װӊĶĖæĶųøō°å£¬ĄūÓĆøĆ×°ÖĆæÉÖĘČ”ÄÄŠ©ĘųĢå£æ
(Š“³öĮ½ÖÖ)”£
(2)Čō½«ĶĖæĶųøō°åøÄĪŖĢśĖæĶųøō°å£¬ŌņøĆ×°ÖĆæÉÓĆÓŚÖĘČ”ŗĪÖÖĘųĢå£æ ”£øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
£Ø10·Ö£©1,2 £¶žäåŅŅĶéæÉ×÷ĘūÓĶæ¹±¬¼ĮµÄĢķ¼Ó¼Į£¬³£ĪĀĻĀĖüŹĒĪŽÉ«ŅŗĢ壬ĆܶČ2.18 g”¤cm£3£¬·Šµć131.4”ę£¬ČŪµć9.79”ę£¬²»ČÜÓŚĖ®£¬Ņ×ČÜÓŚ“¼”¢ĆŃ”¢±ūĶŖµČÓŠ»śČܼĮ”£ŌŚŹµŃéŹŅÖŠæÉŅŌÓĆĻĀĶ¼ĖłŹ¾×°ÖĆÖʱø1,2 £¶žäåŅŅĶ锣ĘäÖŠ·ÖŅŗĀ©¶·ŗĶÉÕĘæa֊װӊŅŅ“¼ŗĶÅØĮņĖįµÄ»ģŗĻŅŗ£¬ŹŌ¹Üd֊װӊŅŗäå(±ķĆęø²øĒÉŁĮæĖ®)”£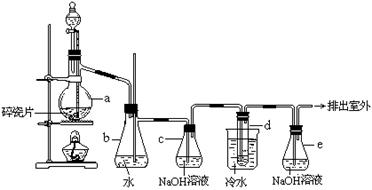
ĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©Š“³ö±¾ĢāÖŠÖʱø1,2-¶žäåŅŅĶéµÄĮ½øö»Æѧ·“Ó¦·½³ĢŹ½”£
______________________ £» ”£
£Ø2£©°²Č«ĘæbæÉŅŌ·ĄÖ¹µ¹Īü£¬²¢æÉŅŌ¼ģ²éŹµŃé½ųŠŠŹ±ŹŌ¹ÜdŹĒ·ń·¢Éś¶ĀČū”£ĒėŠ“³ö·¢Éś¶ĀČūŹ±ĘæbÖŠµÄĻÖĻó£ŗ____________________________________________”£
£Ø3£©ČŻĘ÷cÖŠNaOHČÜŅŗµÄ×÷ÓĆŹĒ£ŗ________________________________________”£
£Ø4£©Ä³Ń§ÉśŌŚ×ö“ĖŹµŃ鏱£¬Ź¹ÓĆŅ»¶ØĮæµÄŅŗä壬µ±äåČ«²æĶŹÉ«Ź±£¬ĖłĻūŗÄŅŅ“¼ŗĶÅØĮņĖį»ģŗĻŅŗµÄĮ棬±ČÕż³£ĒéæöĻĀ³¬¹żŠķ¶ą”£Čē¹ū×°ÖƵÄĘųĆÜŠŌƻӊĪŹĢā£¬ŹŌ·ÖĪöĘäæÉÄܵÄŌŅņ”£__________
______________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
£Ø15·Ö£©Ä³ŃŠ¾æŠ”×éÓĆ»ĘĶæó£ØÖ÷ŅŖ³É·ÖŹĒCuFeS2£¬ĘäÖŠSĪŖ-2¼Ū£©ĪŖÖ÷ŅŖŌĮĻĮ¶Ķ£¬Ęä×Ü·“Ó¦ĪŖ£ŗ2CuFeS2+2SiO2+5O2£½2Cu+2FeSiO3+4SO2”£ŹĀŹµÉĻøĆ·“Ó¦ŹĒ°“ČēĻĀĮ÷³Ģ·Ö²½½ųŠŠµÄ£ŗ

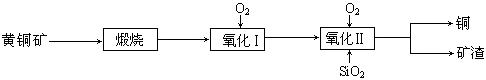
£Ø1£©Ńõ»Æ¢ńµÄ·“Ó¦Ö÷ŅŖŹĒģŃÉÕÉś³ÉµÄĮņ»ÆŃĒĢś±»½ųŅ»²½Ńõ»ÆĪŖŃõ»ÆŃĒĢś£¬²¢Óė¶žŃõ»Æ¹č·“Ӧɜ³ÉæóŌü”£æóŌüµÄÖ÷ŅŖ³É·ÖŹĒ £ØĢī»ÆѧŹ½£©”£
£Ø2£©¾Ż±ØµĄ£¬ÓŠŅ»ÖÖĻø¾śŌŚŃõĘų“ęŌŚĻĀæÉŅŌ½«»ĘĶæóŃõ»Æ³ÉĮņĖįŃĪ£¬·“Ó¦ŹĒŌŚĖįŠŌČÜŅŗÖŠ·¢ÉśµÄ”£øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©ĪŅ¹śŃ§ÕßŃŠ¾æ·¢ĻÖ£¬ŅŌ¾«CuFeS2æóĪŖŌĮĻŌŚ·ŠĢŚĀÆÖŠÓėO2£ØæÕĘų£©·“Ó¦£¬Éś³ÉĪļĄäČ“ŗó¾Čܽā”¢³żĢś”¢½į¾§£¬µĆµ½CuSO4”¤5H2O£¬Éś²ś³É±¾Äܹ»½µµĶŠķ¶ą”£ÓŠ¹ŲŹµŃé½į¹ūČēĻĀ±ķ£ŗ
| ·ŠĢŚĀÆĪĀ¶Č/”ę | 560 | 580 | 600 | 620 | 640 | 660 |
| Ė®ČÜŠŌCu/% | 90.12 | 91.24 | 93.50 | 92.38 | 89.96 | 84.23 |
| ĖįČÜŠŌCu/% | 92.00 | 93.60 | 97.08 | 97.82 | 98.16 | 98.19 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
£Ø12·Ö£©ĮņĖįŠæ±»¹ć·ŗÓ¦ÓĆÓŚ¹¤Å©ŅµÉś²śŗĶŅ½Ņ©ĮģÓņ”£¹¤ŅµÉĻÓÉŃõ»ÆŠææó£ØÖ÷ŅŖ³É·ÖĪŖZnO£¬Įķŗ¬ZnSiO3”¢FeCO3”¢CuOµČ£©Éś²śZnSO4”¤7H2OµÄŅ»ÖÖĮ÷³ĢČēĻĀ£ŗ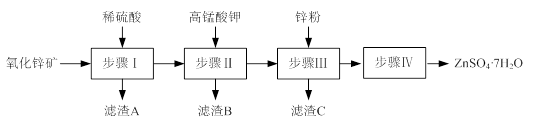
¢Å²½Öč¢ń°üĄØĖį½žŗĶ¹żĀĖĮ½øö²Ł×÷”£
¢ŁĖį½žŹ±£¬Šč²»¶ĻĶØČėøßĪĀĖ®ÕōĘųµÄÄæµÄŹĒ ”£
¢Ś¹żĀĖŹ±£¬ĪŖ·ĄÖ¹¶ĀČū£¬¹żĀĖ×°ÖĆŠč¾³£ÓĆĒāŃõ»ÆÄĘČÜŅŗĒåĻ“£¬ĘäĒåĻ“ŌĄķŹĒ
£ØÓĆ»Æѧ·½³ĢŹ½±ķŹ¾£©”£
¢Ę²½Öč¢ņÖŠ£¬ŌŚpHŌ¼ĪŖ5.1µÄĀĖŅŗÖŠ¼ÓČėøßĆĢĖį¼Ų£¬Éś³ÉFe(OH)3ŗĶMnO(OH)2Į½ÖÖ³Įµķ£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
¢Ē²½Öč¢óĖłµĆĀĖŌüCµÄÖ÷ŅŖ³É·ÖŹĒ ”£
¢ČČ”28.70 g ZnSO4”¤7H2O¼ÓČČÖĮ²»Ķ¬ĪĀ¶Č£¬Ź£Óą¹ĢĢåµÄÖŹĮæ±ä»ÆČēĶ¼ĖłŹ¾”£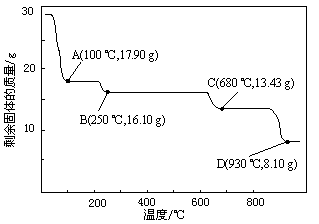
¢Ł²½Öč¢ōÖŠµÄŗęøɲŁ×÷ŠčŌŚ¼õŃ¹Ģõ¼žĻĀ½ųŠŠ£¬ĘäŌŅņŹĒ ”£
¢Ś680 ”ꏱĖłµĆ¹ĢĢåµÄ»ÆѧŹ½ĪŖ ”£
a£®ZnO b£®Zn3O(SO4)2 c£®ZnSO4 d£®ZnSO4”¤H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
£Ø18·Ö£©¼×”¢ŅŅĮ½øöŃŠ¾æŠŌѧĻ°Š”×éĪŖ²ā¶Ø°±·Ö×ÓÖŠµŖ”¢ĒāŌ×ÓøöŹż±Č£¬Éč¼ĘĮĖČēĻĀŹµŃéĮ÷³Ģ£ŗ
ŹµŃéÖŠ£¬ĻČÓĆÖʵƵݱĘųÅž”Ļ“ĘųĘæĒ°ĖłÓŠ×°ÖĆÖŠµÄæÕĘų£¬ŌŁĮ¬½ÓĻ“ĘųĘæŗĶĘųĢåŹÕ¼Æ×° ÖĆ£¬Į¢¼“¼ÓČČŃõ»ÆĶ”£·“Ó¦Ķź³Éŗó£¬ŗŚÉ«Ńõ»ÆĶ×Ŗ»ÆĪŖŗģÉ«µÄĶ”£ĻĀĶ¼A”¢B”¢CĪŖ¼×”¢ŅŅĮ½Š”×éÖĘČ”°±ĘųŹ±æÉÄÜÓƵ½µÄ×°ÖĆ£¬DĪŖŹ¢ÓŠÅØĮņĖįµÄĻ“ĘųĘ攣
¼×Š”×é²āµĆ£ŗ·“Ó¦Ē°Ńõ»ÆĶµÄÖŹĮæm1 g”¢Ńõ»ÆĶ·“Ó¦ŗóŹ£Óą¹ĢĢåµÄÖŹĮæm2g”¢Éś³ÉµŖĘųŌŚ±ź×¼×“æöĻĀµÄĢå»żV1L”£ŅŅŠ”×é²āµĆ£ŗĻ“ĘųĒ°×°ÖĆDµÄÖŹĮæm3g”¢Ļ“Ęųŗó×°ÖĆDµÄÖŹĮæĪŖm4g”¢Éś³ÉµŖĘųŌŚ±ź×¼×“æöĻĀµÄĢå»żV2L”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¼ģ²éA×°ÖĆĘųĆÜŠŌµÄ²Ł×÷ŹĒ____________________________________________________”£
£Ø2£©ŹµŃéŹŅ¼ģŃé°±ĘųµÄ²Ł×÷ŗĶĻÖĻóŹĒ____________________________________”£
£Ø3£©¼×”¢ŅŅĮ½Š”×éŃ”ŌńĮĖ²»Ķ¬µÄ·½·ØÖĘČ”°±Ęų£¬Ēė½«ŹµŃé×°ÖƵÄ×ÖÄø±ąŗÅŗĶÖʱøÖʱøŌĄķĢīŠ“ŌŚĻĀ±ķµÄæÕøńÖŠ”£
| | ŹµŃé×°ÖĆ | ŹµŃéŅ©Ę· | ÖʱøŌĄķ |
| ¼×Š”×é | A | ĒāŃõ»ÆøĘ”¢ĮņĖįļ§ | ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____________ |
| ŅŅŠ”×é | _____ | ÅØ°±Ė®”¢ĒāŃõ»ÆÄĘ | ÓĆ»ÆŃ§Ę½ŗāŌĄķ·ÖĪöĒāŃõ»ÆÄʵÄ×÷ÓĆ£ŗ_______ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
£Ø15·Ö£©»ĘĶæóŹĒ¹¤ŅµĮ¶ĶµÄÖ÷ŅŖŌĮĻ£¬ĘäÖ÷ŅŖ³É·ÖĪŖCuFeS2£¬ĻÖÓŠŅ»ÖÖĢģČ»»ĘĶæó£Øŗ¬ÉŁĮæSiO2£©£¬ĪŖĮĖ²ā¶ØøĆ»ĘĶæóµÄ“æ¶Č£¬Ä³Ķ¬Ń§Éč¼ĘĮĖČēĻĀŹµŃé£ŗ
ĻÖ³ĘȔъĻøµÄ»ĘĶæóѳʷ1.150g£¬ŌŚæÕĘų“ęŌŚĻĀ½ųŠŠģŃÉÕ£¬Éś³ÉCu”¢Fe3O4ŗĶSO2ĘųĢ壬ŹµŃéŗóČ”dÖŠČÜŅŗµÄ ÖĆӌ׶ŠĪĘæÖŠ£¬ÓĆ0.05mol/L±ź×¼µāČÜŅŗ½ųŠŠµĪ¶Ø£¬Ļūŗıź×¼ČÜŅŗ20.00ml”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ÖĆӌ׶ŠĪĘæÖŠ£¬ÓĆ0.05mol/L±ź×¼µāČÜŅŗ½ųŠŠµĪ¶Ø£¬Ļūŗıź×¼ČÜŅŗ20.00ml”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©³ĘĮæѳʷĖłÓƵÄŅĒĘ÷ĪŖ_____(Ģī”°ĶŠÅĢĢģĘ½”±»ņ”°µē×ÓĢģĘ½”±)£¬½«ŃłĘ·ŃŠĻøŗóŌŁ·“Ó¦£¬ĘäÄæµÄŹĒ_______ ”£
£Ø2£©×°ÖĆaŗĶcµÄ×÷ÓĆ·Ö±šŹĒ____ŗĶ____£ØĢī±źŗÅ£©”£
A³żČ„SO2ĘųĢå B³żČ„æÕĘųÖŠµÄĖ®ÕōĘų CÓŠĄūÓŚĘųĢå»ģŗĻ
DÓŠĄūÓŚ¹Ū²ģæÕĘųĮ÷ĖŁ E³żČ„·“Ó¦ŗó¶ąÓąµÄŃõĘų
£Ø3£©ÉĻŹö·“Ó¦½įŹųŗó£¬ČŌŠčĶØŅ»¶ĪŹ±¼äµÄæÕĘų£¬ĘäÄæµÄŹĒ___________”£
£Ø4£©Ķعż¼ĘĖćæÉÖŖ£¬øĆ»ĘĶæóµÄ“æ¶ČĪŖ________”£
£Ø5£©ČōÓĆÓŅĶ¼×°ÖĆĢę“śÉĻŹöŹµŃé×°ÖĆd£¬Ķ¬ŃłæÉŅŌ“ļµ½ŹµŃéÄæµÄµÄŹĒ____£ØĢīŠņŗÅ£©”£
£Ø6£©Čō½«Ō×°ÖĆdÖŠµÄŹŌŅŗøÄĪŖBa(OH)2£¬²āµĆµÄ»ĘĶæó“æ¶ČĪó²īĪŖ£«1%£¬¼ŁÉ菵Ńé²Ł×÷¾łÕżČ·£¬æÉÄܵÄŌŅņÖ÷ŅŖÓŠ_____________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
[14·Ö]ŹµŃéŹŅÖʱø±½ŅŅĶŖµÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
Öʱø¹ż³ĢÖŠ»¹ÓŠ
 µČø±·“Ó¦”£
µČø±·“Ó¦”£
Ö÷ŅŖŹµŃé×°ÖĆŗĶ²½ÖčČēĻĀ£ŗ
£ØI£©ŗĻ³É£ŗŌŚČż¾±ĘæÖŠ¼ÓČė20 gĪŽĖ®AlCl3ŗĶ30 mLĪŽĖ®±½”£ĪŖ±ÜĆā·“Ó¦ŅŗÉżĪĀ¹żæģ£¬±ß½Į°č±ßĀżĀżµĪ¼Ó6 mLŅŅĖįōūŗĶ10 mLĪŽĖ®±½µÄ»ģŗĻŅŗ£¬æŲÖʵĪ¼ÓĖŁĀŹ£¬Ź¹·“Ó¦Ņŗ»ŗ»ŗ»ŲĮ÷”£µĪ¼ÓĶź±Ļŗó¼ÓČČ»ŲĮ÷1Š”Ź±”£
£Ø¢ņ£©·ÖĄėÓėĢį“æ£ŗ
¢Ł±ß½Į°č±ßĀżĀżµĪ¼ÓŅ»¶ØĮæÅØŃĪĖįÓė±łĖ®»ģŗĻŅŗ£¬·ÖĄėµĆµ½ÓŠ»ś²ć
¢ŚĖ®²ćÓƱ½ŻĶČ”£¬·ÖŅŗ
¢Ū½«¢Ł¢ŚĖłµĆÓŠ»ś²ćŗĻ²¢£¬Ļ“µÓ”¢øÉŌļ”¢ÕōČ„±½£¬µĆµ½±½ŅŅĶŖ“Ö²śĘ·
¢ÜÕōĮó“Ö²śĘ·µĆµ½±½ŅŅĶŖ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŅĒĘ÷aµÄĆū³Ę£ŗ____________£»×°ÖĆbµÄ×÷ÓĆ£ŗ________________________________”£
£Ø2£©ŗĻ³É¹ż³ĢÖŠŅŖĒóĪŽĖ®²Ł×÷£¬ĄķÓÉŹĒ____________________________________________”£
£Ø3£©Čō½«ŅŅĖįōūŗĶ±½µÄ»ģŗĻŅŗŅ»“ĪŠŌµ¹ČėČż¾±Ę棬æÉÄܵ¼ÖĀ_________________”£
| A£®·“Ó¦Ģ«¾ēĮŅ | B£®ŅŗĢåĢ«¶ą½Į²»¶Æ | C£®·“Ó¦±ä»ŗĀż | D£®ø±²śĪļŌö¶ą |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
¢ń.(1)ij»ÆѧŠĖȤŠ”×éÓū“ÓĻĀĮŠ×°ÖĆ֊єȔ±ŲŅŖµÄ×°ÖĆÖĘČ”£ØNH4£©2SO4ČÜŅŗ£¬Į¬½ÓµÄĖ³Šņ£ØÓĆ½ÓæŚŠņŗÅ×ÖÄø±ķŹ¾£©ŹĒ£ŗa ”£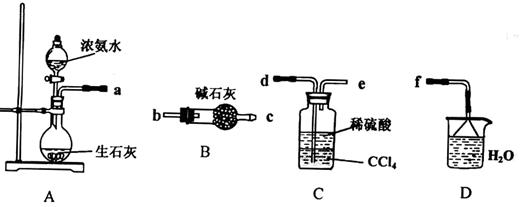
(2)½«×°ÖĆCÖŠĮ½ÖÖŅŗĢå·ÖĄėæŖµÄ²Ł×÷Ćū³ĘŹĒ___ __”£×°ÖĆDµÄ×÷ÓĆŹĒ ”£
¢ņ.¹żŃõ»ÆøĘæÉŅŌÓĆÓŚøÄÉʵŲ±ķĖ®ÖŹ”¢“¦Ąķŗ¬ÖŲ½šŹōĮ£×Ó·ĻĖ®ŗĶÖĪĄķ³ą³±£¬Ņ²æÉÓĆÓŚÓ¦¼±¹©ŃõµČ”£¹¤ŅµÉĻÉś²ś¹żŃõ»ÆøʵÄÖ÷ŅŖĮ÷³ĢČēĻĀ£ŗ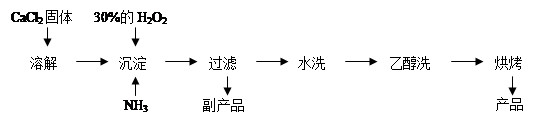
ŅŃÖŖCaO2”¤8H2O³Ź°×É«£¬Ī¢ČÜÓŚĖ®”£I2+2S2O32£= 2I£+S4O62£
£Ø1£©ÓĆÉĻŹö·½·ØÖĘČ”CaO2”¤8H2OµÄ»Æѧ·½³ĢŹ½ŹĒ £»
£Ø2£©¼ģŃé”°Ė®Ļ“”±ŹĒ·ńŗĻøńµÄ·½·ØŹĒ £»
£Ø3£©²ā¶Ø²śĘ·ÖŠCaO2µÄŗ¬ĮæµÄŹµŃé²½ÖčŹĒ£ŗ
µŚŅ»²½£ŗ×¼Č·³ĘČ”a g²śĘ·ÓŚ×¶ŠĪĘæÖŠ£¬¼ÓČėŹŹĮæÕōĮóĖ®ŗĶ¹żĮæµÄb g KI¾§Ģ壬ŌŁµĪČėÉŁĮæ2 mol/LµÄH2SO4ČÜŅŗ£¬³ä·Ö·“Ó¦”£
µŚ¶ž²½£ŗĻņÉĻŹö׶ŠĪĘæÖŠ¼ÓČė¼øµĪµķ·ŪČÜŅŗ”£
µŚČż²½£ŗÖšµĪ¼ÓČėÅضČĪŖc mol”¤L”Ŗ1µÄNa2S2O3ČÜŅŗÖĮ·“Ó¦ĶźČ«£¬ĻūŗÄNa2S2O3ČÜŅŗV mL”£
¢ŁÅŠ¶Ļ“ĖµĪ¶ØŹµŃé“ļµ½ÖÕµćµÄ·½·ØŹĒ£ŗ ”£
¢ŚCaO2µÄÖŹĮæ·ÖŹżĪŖ (ÓĆ×ÖÄø±ķŹ¾)£»
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com