
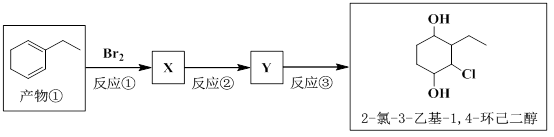
ÓŠ»śĪļA”¢B”¢C”¢D”¢E”¢F”¢GµÄĻą»„¹ŲĻµČēĻĀĶ¼ĖłŹ¾”£
£Ø1£©¼ģŃéAÖŠĀ±ŌŖĖŲµÄŹµŃé·½·ØŹĒ ”£
£Ø2£©BµÄ½į¹¹¼ņŹ½ĪŖ £»¢ŁµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ”£
£Ø3£©GŌŚŹµŃéŹŅÖŠæÉĶعżĮ½²½·“Ó¦Ńõ»Æ³ÉF”£ĘäÖŠµŚŅ»²½·“Ó¦µÄĢõ¼žŹĒ £¬·“Ó¦µĆµ½µÄ²śĪļæÉÄÜÓŠ£ØĢīŠ“½į¹¹¼ņŹ½£© ”£
£Ø4£©FŹĒŅ»ÖÖ¶žŌŖĖį£¬ĖüŌŚŅ»¶ØĢõ¼žĻĀæÉÓėG·“Ӧɜ³Éøß·Ö×Ó»ÆŗĻĪļ£¬øĆøß·Ö×ӵĽį¹¹¼ņŹ½ĪŖ ”£
Š“³ö·“Ó¦¢ÜµÄ»Æѧ·“Ó¦·½³ĢŹ½ ”£
£Ø1£©Č”ÉŁĮæAÓŚŹŌ¹Ü£¬¼ÓČėĒāŃõ»ÆÄĘČÜŅŗ¼ÓČČŅ»¶ĪŹ±¼ä£¬ĄäČ“ŗó¼ÓČėĻ”ĻõĖįÖĮČÜŅŗ³ŹĖįŠŌ£¬µĪ¼ÓĻõĖįŅųČÜŅŗ£¬Čē¹ūÓŠµ»ĘÉ«³Įµķ³öĻÖ£¬ŌņAÖŠŗ¬äåŌŖĖŲ”££Ø2·Ö£©
£Ø2£©H2C=CH-COOH£Ø1·Ö£©£» ĻūČ„£Ø³ż£©·“Ó¦£Ø1·Ö£©
£Ø3£©Cu»ņAg×÷“߻ƼĮ”¢¼ÓČČ£Ø1·Ö£©”£ HO-CH2-CH2-CHO”¢OHC-CH2-CHO£Ø2·Ö£©
£Ø4£©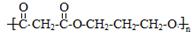 £Ø1·Ö£©
£Ø1·Ö£© +2NaOH
+2NaOH CH2=CHCOONa+HO-CH2-CH2-CH2-OH+NaBr£Ø2·Ö£»·“Ó¦Īļ”¢²śĪļÕżČ·1·Ö£¬ÅäĘ½+Ģõ¼ž1·Ö£©
CH2=CHCOONa+HO-CH2-CH2-CH2-OH+NaBr£Ø2·Ö£»·“Ó¦Īļ”¢²śĪļÕżČ·1·Ö£¬ÅäĘ½+Ģõ¼ž1·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©Ā±“śĢžÖŠĀ±ĖŲĄė×ӵļģŃé·½·ØŹĒ£ŗȔɣĮæAÓŚŹŌ¹Ü£¬¼ÓČėĒāŃõ»ÆÄĘČÜŅŗ¼ÓČČŅ»¶ĪŹ±¼ä£¬ĄäČ“ŗó¼ÓČėĻ”ĻõĖįÖĮČÜŅŗ³ŹĖįŠŌ£¬µĪ¼ÓĻõĖįŅųČÜŅŗ£¬Čē¹ūÓŠµ»ĘÉ«³Įµķ³öĻÖ£¬ŌņAÖŠŗ¬äåŌŖĖŲ”£øł¾ŻAÄÜŌŚĮņĖįµÄĢõ¼žĻĀÉś³ÉĮ½ÖÖĪļÖŹ£¬æÉÖŖŹĒ·¢ÉśĮĖĖ®½ā·“Ó¦£¬Dµ½BŹĒĖį»Æ£¬ĖµĆ÷BŹĒĖį£¬ŌņCŹĒ“¼£¬ĒŅĢ¼Ō×ÓŹżŗĶĢ¼µÄ¹Ē¼ÜĶźČ«ĻąĶ¬”£øł¾Ż×Ŗ»Æ¹ŲĻµæÉÖŖBĪŖH2C=CH-COOH£»FŹĒŅ»ÖÖ¶žŌŖĖį£¬Ķعż×Ŗ»Æ¹ŲĻµæÉµĆ³öCĪŖBrH2C-CH2-CH2OH£¬EĪŖBrH2C-CH2-CHO£¬DĪŖH2C=CH-COONa£¬GĪŖHOH2C-CH2-CH2OH,FĪŖHOOC-CH2-COOH”£
£Ø2£©BµÄ½į¹¹¼ņŹ½ĪŖH2C=CH-COOH”£¢ŁµÄ»Æѧ·“Ó¦ĄąŠĶŹĒĻūČ„£Ø³ż£©·“Ó¦”£GŌŚŹµŃéŹŅÖŠæÉĶعżĮ½²½·“Ó¦Ńõ»Æ³ÉF”£ĘäÖŠµŚŅ»²½·“Ó¦µÄĢõ¼žŹĒCu»ņAg×÷“߻ƼĮ”¢¼ÓČČ£¬·“Ó¦µĆµ½µÄ²śĪļÓŠ²æ·ÖŃõ»Æ»ņČ«²æŃõ»Æ£¬¹Ź²śĪļæÉÄÜÓŠHO-CH2-CH2-CHO”¢OHC-CH2-CHO”£
£Ø4£©FŹĒŅ»ÖÖ¶žŌŖĖį£¬ĖüŌŚŅ»¶ØĢõ¼žĻĀæÉÓėG·“Ӧɜ³Éøß·Ö×Ó»ÆŗĻĪļ£¬Ļąµ±ÓŚ·¢ÉśĮĖõ„»Æ·“Ӧɜ³ÉĮĖøß¾ŪĪļ”£Š“³ö·“Ó¦¢ÜĪŖõ„ŗĶĀ±“śĢžµÄĖ®½ā·“Ó¦£¬ÓŠĮ½øö¹ŁÄÜĶÅ·¢ÉśĮĖ±ä»Æ”£æ¼µć£ŗ±¾Ģāæ¼²éĮĖÓŠ»śæņĶ¼ĶʶĻ£¬ŹĒŅ»ÖÖ×ŪŗĻŠŌµÄĒ°ŗóĶʶĻ£¬ÄŃ¶Č½Ļ“ó”£


 ÖŠæ¼½ā¶Įæ¼µć¾«Į·ĻµĮŠ“š°ø
ÖŠæ¼½ā¶Įæ¼µć¾«Į·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
»ÆŗĻĪļF(Ę„·„ĖūĶ”)ÓĆÓŚøߵعĢ“¼ŃŖÖ¢µÄÖĪĮĘ£¬ĘäŗĻ³ÉĀ·ĻßČēĻĀ£ŗ
(1)»ÆŗĻĪļDÖŠ¹ŁÄÜĶŵÄĆū³ĘĪŖ________”¢________ŗĶõ„¼ü”£
(2)A”śBµÄ·“Ó¦ĄąŠĶŹĒ____________”£
(3)Š“³öĶ¬Ź±Āś×ćĻĀĮŠĢõ¼žµÄAµÄŅ»ÖÖĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£ŗ________________”£
¢Ł·Ö×ÓÖŠŗ¬ÓŠĮ½øö±½»·£»¢Ś·Ö×ÓÖŠÓŠ3ÖÖ²»Ķ¬»Æѧ»·¾³µÄĒā£»¢Ū²»ŗ¬”ŖO”ŖO”Ŗ”£
(4)ŹµĻÖD”śEµÄ×Ŗ»ÆÖŠ£¬»ÆŗĻĪļXµÄ·Ö×ÓŹ½ĪŖC19H15NFBr£¬Š“³öĘä½į¹¹¼ņŹ½£ŗ________________”£
(5)ŅŃÖŖ£ŗ»ÆŗĻĪļEŌŚCF3COOH“ß»Æ×÷ÓĆĻĀĻČ×Ŗ»ÆĪŖ £¬ŌŁ×Ŗ»ÆĪŖF”£ÄćČĻĪŖŗĻ³ÉĀ·ĻßÖŠÉč¼Ę²½Öč¢ŚµÄÄæµÄŹĒ___________________________________________________”£
£¬ŌŁ×Ŗ»ÆĪŖF”£ÄćČĻĪŖŗĻ³ÉĀ·ĻßÖŠÉč¼Ę²½Öč¢ŚµÄÄæµÄŹĒ___________________________________________________”£
(6)ÉĻŹöŗĻ³ÉĀ·ĻßÖŠ£¬²½Öč¢ŪµÄ²śĪļ³żDĶā»¹Éś³É £¬øĆ·“Ó¦ŌĄķŌŚÓŠ»śŗĻ³ÉÖŠ¾ßÓŠ¹ć·ŗÓ¦ÓĆ”£ŹŌŠ“³öŅŌ
£¬øĆ·“Ó¦ŌĄķŌŚÓŠ»śŗĻ³ÉÖŠ¾ßÓŠ¹ć·ŗÓ¦ÓĆ”£ŹŌŠ“³öŅŌ ĪŖÖ÷ŅŖŌĮĻÖʱø
ĪŖÖ÷ŅŖŌĮĻÖʱø µÄŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼(ĪŽ»śŹŌ¼ĮČĪÓĆ)”£ŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼Ź¾ĄżČēĻĀ£ŗ
µÄŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼(ĪŽ»śŹŌ¼ĮČĪÓĆ)”£ŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼Ź¾ĄżČēĻĀ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ä³Ń§Ļ°Š”×éĪŖĢ½¾æĪ¬ÉśĖŲCµÄ×é³ÉŗĶÓŠ¹ŲŠŌÖŹ£¬½ųŠŠĮĖŅŌĻĀŹµŃé£ŗ
¢ŁČ”Ī¬ÉśĖŲCѳʷъĖ飬£®³ĘČ”øĆŹŌŃł0.704 g£¬ÖĆÓŚ²¬ÖŪ²¢·ÅČĖČ¼ÉÕ¹ÜÖŠ£¬²»¶ĻĶØČėŃõĘųĮ÷”£ÓĆ¾Ę¾«ÅēµĘ³ÖŠų¼ÓČČѳʷ£¬ŃłĘ·Öš½„ĻūŹ§×īŗóĪŽČĪŗĪ²ŠĮōĪļ£¬½«Éś³ÉĪļ(½öÓŠĮ½ÖÖĪļÖŹ)ĻČŗóĶعżĪŽĖ®ĮņĖįĶŗĶ¼īŹÆ»Ņ£¬Į½Õß·Ö±šŌöÖŲ0.288 gŗĶ1.056 g£¬Éś³ÉĪļĶźČ«±»ĪüŹÕ”£
¢Ś½«ÉŁŠķŃŠĖéµÄĪ¬ÉśĖŲCѳʷŗĶŹŹĮæµÄ×ĻÉ«ŹÆČļŹŌŅŗ³ä·Ö»ģŗĻŗó£¬ČÜŅŗŃÕÉ«±äŗģ”£
Ēė½ā“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©³ĘČ”µÄŹŌŃłÖŠ£¬ŗ¬ÓŠĒāŌ×ÓµÄĪļÖŹµÄĮæŹĒ mo1£»Ģ¼ŌŖĖŲµÄÖŹ×īŹĒ g”£
£Ø2£©Ī¬ÉśĖŲCÖŠ (Ģī”°ŗ¬”±”¢”°²»ŗ¬”±»ņ”°ĪŽ·ØČ·¶ØŹĒ·ń”±)ÓŠŃõŌŖĖŲ£¬ĄķÓÉŹĒøł¾ŻŹµŃ鏿¾ŻæÉÖŖ ”£
ČēÄć»Ų“š”°ŗ¬”±ÓŠ£¬Ēė¼ĘĖć³ĘČ”µÄŹŌŃłÖŠŃõŌ×ÓµÄĪļÖŹµÄĮæ²¢ĢīČėĻĀŅ»æÕøńÖŠ£»ČēÄć»Ų“š”°²»ŗ¬”±µČ£¬ŌņĻĀŅ»æÕøń²»±ŲĢīŠ“”£³ĘČ”ŹŌŃłÖŠŃõŌ×ÓµÄĪļÖŹµÄĮæĪŖ mol”£
£Ø3£©Čē¹ūŅŖČ·¶ØĪ¬ÉśĖŲCµÄ·Ö×ÓŹ½£¬ÄćČĻĪŖ»¹ŠčŅŖÖŖµĄµÄŠÅĻ¢ŹĒ ”£
£Ø4£©Ī¬ÉśĖŲCŗĶ×ĻÉ«ŹÆČļŹŌŅŗ»ģŗĻŗó£¬ČÜŅŗŃÕÉ«±äŗģ£¬ĖµĆ÷Ī¬ÉśĖŲCČÜŅŗ¾ßÓŠ (Ģī”°Ėį”±”¢”°¼ī”±»ņ”°ÖŠ”±)ŠŌ”£²éŌÄÓŠ¹Ų׏ĮĻĻŌŹ¾£¬Ī¬ÉśĖŲC¾ßÓŠ»¹ŌŠŌ”£ĒėŅŌµķ·ŪČÜŅŗ”¢µāĖ®ĪŖŹŌ¼Į£¬Š“³öŃéÖ¤Ī¬ÉśĖŲC¾ßÓŠ»¹ŌŠŌµÄŹµŃé·½·ØŗĶĻÖĻó£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ļć¶¹ĖŲŹĒÓĆĶ¾¹ć·ŗµÄĻćĮĻ£¬ŗĻ³ÉĻć¶¹ĖŲµÄĀ·ĻßČēĻĀ£ØĘäĖūŹŌ¼Į”¢²śĪļ¼°·“Ó¦Ģõ¼ž¾łŹ”ĀŌ£©£ŗ
£Ø1£©¢ńµÄ·Ö×ÓŹ½ĪŖ £»
£Ø2£©·“Ó¦¢ŚµÄ·“Ó¦ĄąŠĶŹĒ £¬·“Ó¦¢ÜµÄ·“Ó¦ĄąŠĶŹĒ ”£
£Ø3£©Ļć¶¹ĖŲŌŚ¹żĮæNaOHČÜŅŗÖŠĶźČ«Ė®½āµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©¢õŹĒ¢ōµÄĶ¬·ÖŅģ¹¹Ģ壬¢õµÄ·Ö×ÓÖŠŗ¬ÓŠ±½»·ĒŅĪŽĢ¼Ģ¼Ė«¼ü£¬±½»·ÉĻŗ¬ÓŠĮ½øöĮŚĪ»Č”“ś»ł£¬ÄÜ·¢ÉśŅų¾µ·“Ó¦”£¢õµÄ½į¹¹¼ņŹ½ĪŖ £ØČĪŠ“Ņ»ÖÖ£©”£
£Ø5£©Ņ»¶ØĢõ¼žĻĀ£¬ ÓėCH3CHOÄÜ·¢ÉśĄąĖĘ·“Ó¦¢Ł”¢¢ŚµÄĮ½²½·“Ó¦£¬×īÖÕÉś³ÉµÄÓŠ»śĪļµÄ½į¹¹¼ņŹ½ĪŖ ”£
ÓėCH3CHOÄÜ·¢ÉśĄąĖĘ·“Ó¦¢Ł”¢¢ŚµÄĮ½²½·“Ó¦£¬×īÖÕÉś³ÉµÄÓŠ»śĪļµÄ½į¹¹¼ņŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĢžAÓėĒāĘųŅŌĪļÖŹµÄĮæ1:1·¢Éś¼Ó³É·“Ó¦Ź±æÉŅŌµĆµ½¶ąÖÖ²śĪļ£¬ŅŌĻĀŹĒĘäÖŠĮ½ÖÖ²śĪļµÄ½į¹¹¼ņŹ½£ŗ
| ²śĪļ | ¢Ł | ¢Ś |
| ½į¹¹¼ņŹ½ | 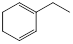 | 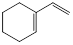 |
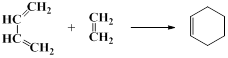
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ļą¶Ō·Ö×ÓÖŹĮæĪŖ92µÄij·¼ĻćĢžXŹĒŅ»ÖÖÖŲŅŖµÄÓŠ»ś»Æ¹¤ŌĮĻ£¬ŃŠ¾æ²æĆÅŅŌĖüĪŖ³õŹ¼ŌĮĻÉč¼Ę³öČēĻĀ×Ŗ»Æ¹ŲĻµĶ¼£Ø²æ·Ö²śĪļ”¢ŗĻ³ÉĀ·Ļß”¢·“Ó¦Ģõ¼žĀŌČ„£©”£ĘäÖŠAŹĒŅ»ĀČ“śĪļ£¬HŹĒŅ»ÖÖ¹¦ÄÜøß·Ö×Ó£¬Į“½Ś×é³ÉĪŖC7H5NO”£
ŅŃÖŖ£ŗ
¢ń”¢
¢ņ”¢
Ēėøł¾ŻĖłŃ§ÖŖŹ¶Óė±¾ĢāĖłøųŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©HµÄ½į¹¹¼ņŹ½ŹĒ£ŗ £»X”śAµÄ·“Ó¦Ģõ¼ž ”£
£Ø2£©·“Ó¦¢ŚµÄĄąŠĶŹĒ £»·“Ó¦¢ŚŗĶ¢ŪĻČŗóĖ³Šņ²»Äܵߵ¹µÄŌŅņŹĒ £»
£Ø3£©Š“³ö·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½£ŗ £»
£Ø4£© ÓŠ¶ąÖÖĶ¬·ÖŅģ¹¹Ģ壬Š“³ö2ÖÖŗ¬ÓŠ1øöČ©»łŗĶ2øöōĒ»łĒŅ±½»·ÉĻÖ»ÓŠ2ÖÖŅ»ĀČČ”“śĪļµÄ·¼Ļć×å»ÆŗĻĪļµÄ½į¹¹¼ņŹ½£ŗ £»
ÓŠ¶ąÖÖĶ¬·ÖŅģ¹¹Ģ壬Š“³ö2ÖÖŗ¬ÓŠ1øöČ©»łŗĶ2øöōĒ»łĒŅ±½»·ÉĻÖ»ÓŠ2ÖÖŅ»ĀČČ”“śĪļµÄ·¼Ļć×å»ÆŗĻĪļµÄ½į¹¹¼ņŹ½£ŗ £»
£Ø5£©Š“³öÓÉA×Ŗ»ÆĪŖ µÄĀ·Ļß”££ØÓĆA”ś ”” ”ś
µÄĀ·Ļß”££ØÓĆA”ś ”” ”ś ŌŚ¼żŗÅÉĻŠ“Ć÷·“Ó¦ŹŌ¼Į¼°·“Ó¦Ģõ¼ž”££©
ŌŚ¼żŗÅÉĻŠ“Ć÷·“Ó¦ŹŌ¼Į¼°·“Ó¦Ģõ¼ž”££©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
»ÆŗĻĪļA£ØC11H8O4£©ŌŚĒāŃõ»ÆÄĘČÜŅŗÖŠ¼ÓČČ·“Ó¦ŗóŌŁĖį»ÆæɵƵ½»ÆŗĻĪļBŗĶC”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©BµÄ·Ö×ÓŹ½ĪŖC2H4O2£¬·Ö×ÓÖŠÖ»ÓŠŅ»øö¹ŁÄÜĶÅ”£ŌņBµÄ½į¹¹¼ņŹ½ŹĒ________£¬BÓėŅŅ“¼ŌŚÅØĮņĖį“ß»ÆĻĀ¼ÓČČ·“Ӧɜ³ÉD£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ________________________£¬øĆ·“Ó¦µÄĄąŠĶŹĒ________£»
£Ø2£©Š“³öĮ½ÖÖÄÜ·¢ÉśŅų¾µ·“Ó¦µÄBµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½________________________”£
£Ø3£©CŹĒ·¼Ļć»ÆŗĻĪļ£¬Ļą¶Ō·Ö×ÓÖŹĮæ180£¬ĘäĢ¼µÄÖŹĮæ·ÖŹżĪŖ60.0£„£¬ĒāµÄÖŹĮæ·ÖŹżĪŖ4.4£„£¬ĘäÓąĪŖŃõ£¬ŌņCµÄ·Ö×ÓŹ½ŹĒ________”£
£Ø4£©ŅŃÖŖCµÄ·¼»·ÉĻÓŠČżøöČ”“ś»ł”£ĘäÖŠŅ»øöČ”“ś»łĪŽÖ§Į“£¬ĒŅÓŠÄÜŹ¹äåµÄĖÄĀČ»ÆĢ¼ČÜŅŗĶŹÉ«µÄ¹ŁÄÜĶż°ÄÜÓėĢ¼ĖįĒāÄĘČÜŅŗ·“Ó¦·Å³öĘųĢåµÄ¹ŁÄÜĶÅ£¬ŌņøĆČ”“ś»łÉĻµÄ¹ŁÄÜĶÅĆū³ĘŹĒ ”£ĮķĶāĮ½øöČ”“ś»łĻąĶ¬£¬·Ö±šĪ»ÓŚøĆČ”“ś»łµÄĮŚĪ»ŗĶ¶ŌĪ»£¬ŌņCµÄ½į¹¹¼ņŹ½ŹĒ ”£
£Ø5£©AµÄ½į¹¹¼ņŹ½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŅĻ©ŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬ŅŌŅŅĻ©ĪŖŌĮĻŃÜÉś³ö²æ·Ö»Æ¹¤²śĘ·µÄ·“Ó¦ČēĻĀ£Ø²æ·Ö·“Ó¦Ģõ¼žŅŃĀŌČ„£©£ŗ
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öAŌŚŹµŃéŹŅÖĘŅŅĻ©µÄ·½³ĢŹ½£ŗ ”£
£Ø2£©BŗĶE·“Ӧɜ³ÉFµÄ»Æѧ·½³ĢŹ½ĪŖ______ _____£¬øĆ·“Ó¦µÄĄąŠĶĪŖ_____________£»
£Ø3£©DµÄ½į¹¹¼ņŹ½ĪŖ____ _______£»
£Ø4£©Š“³öDµÄĶ¬·ÖŅģ¹¹Ģå£Øŗ¬Č©»ł£©·¢ÉśŅų¾µ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø5£©CµÄĶ¬·ÖŅģ¹¹ĢåÖŠŗ¬Č©»łµÄ½į¹¹ÓŠ ÖÖ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
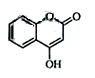 ŹĒŅ»ÖÖŅ½Ņ©ÖŠ¼äĢ壬³£ÓĆĄ“Öʱøæ¹ÄżŃŖŅ©£¬æÉĶعżĻĀĮŠĀ·ĻßŗĻ³É£ŗ
ŹĒŅ»ÖÖŅ½Ņ©ÖŠ¼äĢ壬³£ÓĆĄ“Öʱøæ¹ÄżŃŖŅ©£¬æÉĶعżĻĀĮŠĀ·ĻßŗĻ³É£ŗ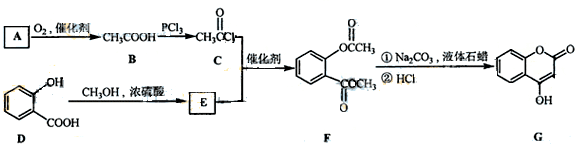
£Ø1£©AÓėŅų°±ČÜŅŗ·“Ó¦ÓŠŅų¾µÉś³É£¬ŌņAµÄ½į¹¹¼ņŹ½ŹĒ ”£
£Ø2£©B”śCµÄ·“Ó¦ĄąŠĶŹĒ ”£
£Ø3£©EµÄ½į¹¹¼ņŹ½ŹĒ
£Ø4£©Š“³öFŗĶ¹żĮæNaOHČÜŅŗ¹²ČČŹ±·“Ó¦µÄ»Æѧ·½³ĢŹ½
£Ø5£©ĻĀĮŠ¹ŲÓŚGµÄĖµ·ØÕżČ·µÄŹĒ ”£
a£®ÄÜÓėäåµ„ÖŹ·“Ó¦ b£®ÄÜÓė½šŹōÄĘ·“Ó¦
c£®1molG×ī¶ąÄÜŗĶ3molĒāĘų·“Ó¦ d£®·Ö×ÓŹ½ŹĒC9H6O3
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com