
��25mL 0.1mol/L NaOH��Һ����μ���0.2mol/L������Һ����������ͼ��ʾ���й�����Ũ�ȹ�ϵ�Ƚϲ���ȷ���� �� ��
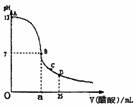
A����A��B����һ�㣨������A��B���㣩����Һ��һ�����У�c��Na+��>c(CH3COO�D )>c(OH�D )>c(H+)
B����B�㣬a>12.5�����У�c��Na+��=c(CH3COO�D)>c(OH�D)=(H+)
C����C�㣺c(CH3COO�D)> c��Na+��>(H+)>c(OH�D)
D����D�㣺c(CH3COO�D)+c(CH3COOH)=2 c��Na+��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��25mL 0.1mol/L NaOH��Һ����μ���0.2mol/L���ᣬ��������ͼ��ʾ���й�����������ǣ�������
��25mL 0.1mol/L NaOH��Һ����μ���0.2mol/L���ᣬ��������ͼ��ʾ���й�����������ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?ӥ̶��ģ�������£���25mL 0.1mol��L-1NaOH��Һ����μ���0.2mol?L-1 CH3COOH ��Һ��pH ��μ� CH3COOH��Һ����Ĺ�ϵ������ͼ��ʾ������������Һ���ʱ������仯�������й�����Ũ�ȹ�ϵ��˵��������ǣ�������
��2011?ӥ̶��ģ�������£���25mL 0.1mol��L-1NaOH��Һ����μ���0.2mol?L-1 CH3COOH ��Һ��pH ��μ� CH3COOH��Һ����Ĺ�ϵ������ͼ��ʾ������������Һ���ʱ������仯�������й�����Ũ�ȹ�ϵ��˵��������ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ƿ�е���Һ | �ζ����е���Һ | ѡ��ָʾ�� | ѡ�õζ��� | |
| A | �� | �� | ʯ�� | �� |
| B | �� | �� | ���� | �� |
| C | �� | �� | ��̪ | �� |
| D | �� | �� | ��̪ | �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��25mL 0.1mol?L-1��NaOH��Һ����μ���0.2mol?L-1��CH3COOH��Һ����ҺpH�仯������ͼ��ʾ��
��25mL 0.1mol?L-1��NaOH��Һ����μ���0.2mol?L-1��CH3COOH��Һ����ҺpH�仯������ͼ��ʾ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com