
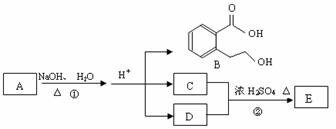
��ͼ�� A��B��C��D��E��Ϊ�л��������֪��C�ܸ�NaHCO3������Ӧ���úͣĵ���Է���������ȣ�
��EΪ��֧���Ļ����
������ͼ�ش����⣺
����1����֪E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%������Ϊ������E�ķ���ʽΪ������������������ ���� C�����еĹ����������� ______________��������B���������ķ�Ӧ�������������������� ������ĸ��ţ���
a���ӳɷ�Ӧ ������b��ȡ����Ӧ ��������c����ȥ��Ӧ
d��������Ӧ ������e��ˮ�ⷴӦ ��������f�� �û���Ӧ
��2����Ӧ�ڵĻ�ѧ����ʽ��_________________���������������������������� _��
��3��A�Ľṹ��ʽ�� __________________������������������ ��
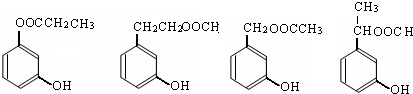
��4��ͬʱ������������������B��ͬ���칹�����Ŀ���������� ����
���м��ȡ�������ṹ
�����ڷ������γɵ���
���� FeCl3 ��Һ������ɫ��Ӧ��
д��������������ͬ���칹��Ľṹ��ʽ������������ �������� ������������ ��
 �ŵ������ϵ�д�
�ŵ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ũ���� |
| �� |
| Ũ���� |
| �� |


 ����1��
����1�� ����1��
����1���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ŨH2SO4 |
| �� |
| ŨH2SO4 |
| �� |




| ���� |

| ���� |
| ���� |

| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
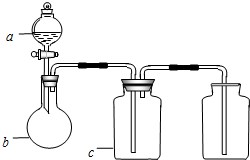 ��ͼ��a��b��c��ʾ��Ӧ�����м�����Լ���������ͼװ����ȡ���������ռ��������ǣ�������
��ͼ��a��b��c��ʾ��Ӧ�����м�����Լ���������ͼװ����ȡ���������ռ��������ǣ�������
| ��� | ���� | a | b | c |
| A | NH3 | Ũ��ˮ | ��ʯ�� | ��ʯ�� |
| B | CO2 | ���� | ̼��� | ����NaHCO3 |
| C | NO | ϡHNO3 | ͭм | H2O |
| D | NO2 | ŨHNO3 | ͭм | NaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ŨH2SO4 |
| �� |
| ŨH2SO4 |
| �� |


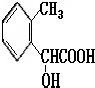

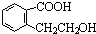
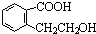
| ���� |
 CH2-CH2
CH2-CH2 n��CH2=CH2+H2O
n��CH2=CH2+H2O| ���� |
| ���� |
 CH2-CH2
CH2-CH2 n��CH2=CH2+H2O
n��CH2=CH2+H2O| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com