

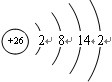
 ���ʴ�Ϊ���������� �ڢ�����
���ʴ�Ϊ���������� �ڢ����� 
 4Al2O3+9Fe��ʵ�����������÷�Ӧ�IJ����������������ȼ������һ������أ���һ��þ������ȼþ����
4Al2O3+9Fe��ʵ�����������÷�Ӧ�IJ����������������ȼ������һ������أ���һ��þ������ȼþ���� 4Al2O3+9Fe���������ȼ������һ������أ���һ��þ������ȼþ����
4Al2O3+9Fe���������ȼ������һ������أ���һ��þ������ȼþ���� Fe3O4+4H2����Ӧ�����ӷ���ʽ��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O��
Fe3O4+4H2����Ӧ�����ӷ���ʽ��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O�� Fe3O4+4H2��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O��
Fe3O4+4H2��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O�� CaCl2+2NH3��+2H2O�������Ǽ�����������ü�ʯ�Ҹ��
CaCl2+2NH3��+2H2O�������Ǽ�����������ü�ʯ�Ҹ�� CaCl2+2NH3��+2H2O����ʯ�ң�
CaCl2+2NH3��+2H2O����ʯ�ң� ��������Һ���������ʵ���Ϊ
��������Һ���������ʵ���Ϊ ����ҺŨ��=
����ҺŨ��= =0.045mol/L���ʴ�Ϊ��0.045mol/L��
=0.045mol/L���ʴ�Ϊ��0.045mol/L��

 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
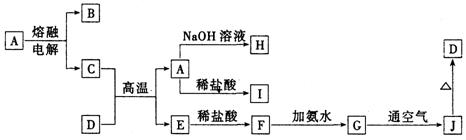
A��J����ѧ��ѧ���������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������֪A��һ�ָ��۵����ʣ�D��һ�ֺ���ɫ���塣
��ش��������⣺
��1��A���ʵ�����Ϊ___________��H��I��Ӧ���������ﻯѧʽΪ ��
��2��C��D�ڸ����µķ�Ӧ��ұ��ҵ�ϳ�Ϊ ��Ӧ�������÷�Ӧ��ʵ�������__________ ______��
��3��G��J�Ļ�ѧ����ʽΪ_________________ __________ _____��
��4��A��H�����ӷ���ʽΪ_________ ____ _______��
��5�������ӷ���ʽ��ʾI���������ھ�ˮ��ԭ��_________ _______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�A��B��C��D��E����ѧ��ѧ������5�ֻ��������A��B�����������X��Y�������г����Ľ�����������ʼ��ת����ϵ����ͼ��ʾ�����ַ�Ӧ�����������ȥ����
��1�����Լ�l���Լ�2������ͬ�����ʣ���X���Լ�l��Ӧ�����ӷ���ʽ�� ��
��2�����Լ�1���Լ�2��ͬ����E��Һ�������ɲ����պ�ɵõ�A����A�Ļ�ѧʽ�� ��
�ټ�������D����Һ�н������ӵ�ʵ������� ��
�ڽ�����C����ˮ������Һ�� ������ԡ��������ԡ����ԡ�����ԭ�������ӷ���ʽ�ɱ�ʾΪ ��
��3����E��Һ���������������ɺ�ɵõ�����Һ�����ʣ���ҵ����E��ϡ�����NaNO2Ϊԭ�����Ʊ���Ч��ˮ��Y��OH��SO4����Ӧ����N0���ɣ��÷�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ̨����ѧ������ѧ�ڵڶ���ͳ����ѧ�Ծ� ���ͣ������
��14�֣�A��B��C��D��E����ѧ��ѧ������5�ֻ��������A��B�����������X��Y�������г����Ľ�����������ʼ��ת�� ��ϵ����ͼ��ʾ�����ַ�Ӧ�����������ȥ����
��ϵ����ͼ��ʾ�����ַ�Ӧ�����������ȥ����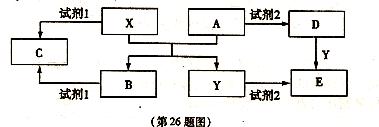
��1�����Լ�l���Լ�2������ͬ�����ʣ���X���Լ�l��Ӧ�����ӷ���ʽ��  ��
��
��2�����Լ�1���Լ�2��ͬ����E��Һ�������ɲ����պ�ɵõ�A����A�Ļ�ѧʽ�� ��
�ټ�������D����Һ�н����� �ӵ�ʵ������� ��
�ӵ�ʵ������� ��
�ڽ�����C����ˮ������Һ�� ������ԡ��������ԡ����ԡ�����ԭ�������ӷ���ʽ�ɱ�ʾΪ ��
��3����E��Һ���������������ɺ�ɵõ�����Һ�����ʣ���ҵ����E��ϡ�����NaNO2Ϊԭ�����Ʊ���Ч��ˮ��Y��OH��SO4����Ӧ����N0���ɣ��÷�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�ڵڶ���ͳ����ѧ�Ծ� ���ͣ������
��14�֣�A��B��C��D��E����ѧ��ѧ������5�ֻ��������A��B�����������X��Y�������г����Ľ�����������ʼ��ת����ϵ����ͼ��ʾ�����ַ�Ӧ�����������ȥ����
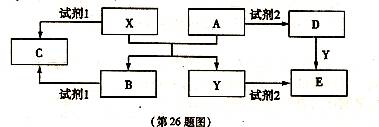
��1�����Լ�l���Լ�2������ͬ�����ʣ���X���Լ�l��Ӧ�����ӷ���ʽ�� ��
��2�����Լ�1���Լ�2��ͬ����E��Һ�������ɲ����պ�ɵõ�A����A�Ļ�ѧʽ�� ��
�ټ�������D����Һ�н������ӵ�ʵ������� ��
�ڽ�����C����ˮ������Һ�� ������ԡ��������ԡ����ԡ�����ԭ�������ӷ���ʽ�ɱ�ʾΪ ��
��3����E��Һ���������������ɺ�ɵõ�����Һ�����ʣ���ҵ����E��ϡ�����NaNO2Ϊԭ�����Ʊ���Ч��ˮ��Y��OH��SO4����Ӧ����N0���ɣ��÷�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com