
| �ζ����� ʵ������ | 1 | 2 | 3 | 4 |
| V������Һ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.00 | 0.200 | 0.10 | 0.00 |
| V��NaOH��/mL���ն����� | 14.95 | 15.20 | 15.15 | 16.95 |
| V��NaOH��/mL�����ģ� | 14.95 | 15.00 | 15.05 | 16.95 |
���� ������Ժ���������֮�䷢���кͷ�Ӧ���ɴ����ƺ�ˮ��
��1������һ�����һ�����ʵ���Ũ�ȵ���Һ��Ҫ�Ķ�������������ƿ��
��2���������Ƶζ�������Һ��Ҫ�÷�̪��ָʾ����
��4���ζ�ʱһ�ֿ��Ƶζ��ܣ�һ��ҡ����ƿ���۾��۲���ƿ����ɫ�ı仯��ֱ���ζ��յ㣻����������ʹ��̪��ʾ��ɫ��
��5��ÿһ�εζ����ĵı�Һ�����������ȣ��������̫��
��6������NaOH��Һ��ƽ��ֵ�Ǹ�����Чֵ�ĺͺ���Чʵ������ı�ֵ��
��7������c�����⣩=$\frac{c��������V������}{V�����⣩}$���������Һ��Ũ�ȣ�
��� �⣺������Ժ���������֮�䷢���кͷ�Ӧ���ɴ����ƺ�ˮ����Ӧ��ԭ��Ϊ��CH3COOH+NaOH=CH3COONa+H2O��
��1������һ�����һ�����ʵ���Ũ�ȵ���Һ��Ҫ�Ķ�������������ƿ���ʴ�Ϊ��100ml����ƿ��
��2���������Ƶζ�������Һ��Ҫ�÷�̪��ָʾ�����ζ��յ�ʱ����Һ����ɫ��Ϊdz��ɫ��������ڲ���ɫ���ʴ�Ϊ��0.1%��̪��Һ��
��4���ζ�ʱһ�ֿ��Ƶζ��ܣ�һ��ҡ����ƿ���۾��۲���ƿ����ɫ�ı仯��ֱ���ζ��յ㣬����������ʹ��̪��ʾ��ɫ�����Եζ��յ�ʱ����Һ����ɫ��Ϊdz��ɫ��������ڲ���ɫ���ʴ�Ϊ����ƿ����Һ��ɫ�仯������ɫ��Ϊdz��ɫ��������ڲ���ɫ��
��5��ÿһ�εζ����ĵı�Һ�����������ȣ��������̫�����ݿ��Կ�������4���������������ʴ�Ϊ��4��
��6������NaOH��Һ��ƽ��ֵ�Ǹ�����Чֵ�ĺͺ���Чʵ������ı�ֵ����$\frac{14.95+15.00+15.05}{3}$mL=15.00mL���ʴ�Ϊ��15.00��
��7��c�����⣩=$\frac{c��������V������}{V�����⣩}$=$\frac{0.0100mol/L��15.00mL}{20.00mL}$=0.075 mol/L���ʴ�Ϊ��0.075 mol/L��
���� �����Բⶨ���۰״�������Ϊ�����������к͵ζ��������������Լ���ѧ���㣬�ѶȲ���ֻҪ���ն�Ӧ֪ʶ������ɣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
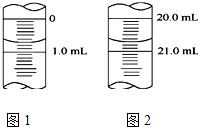 ijѧ����0.1000 mol•L-1KOHҺ�ζ�δ֪Ũ�ȵĴ��ᣬ������ֽ�Ϊ���¼�����
ijѧ����0.1000 mol•L-1KOHҺ�ζ�δ֪Ũ�ȵĴ��ᣬ������ֽ�Ϊ���¼�����| ʵ����� | 1 | 2 | 3 | 4 |
| ����NaOH��Һ�������mL�� | 20.05 | 20.00 | 18.80 | 19.95 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ���й��������£�
�屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ���й��������£�| �� | �� | �屽 | |
| �ܶ�/g•cm-3 | 0.88 | 3.10 | 1.50 |
| �е�/��C | 80 | 59 | 156 |
| ˮ���ܽ�� | �� | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
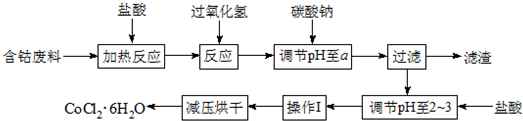
| ������ | Fe��OH��3 | Fe��OH��2 | Co��OH��2 | Al��OH��3 |
| ��ʼ���� | 2.3 | 7.5 | 7.6 | 3.4 |
| ��ȫ���� | 4.1 | 9.7 | 9.2 | 5.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
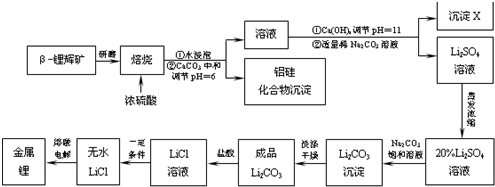
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
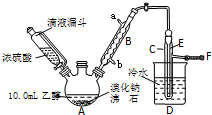 ʵ�������Ҵ���Ũ������廯�Ʒ�Ӧ���Ʊ������飬�䷴Ӧԭ����ʵ���װ�����£���Ӧ��Ҫ���ȣ�ͼ��ʡȥ�˼���װ�ã���
ʵ�������Ҵ���Ũ������廯�Ʒ�Ӧ���Ʊ������飬�䷴Ӧԭ����ʵ���װ�����£���Ӧ��Ҫ���ȣ�ͼ��ʡȥ�˼���װ�ã���| �Ҵ� | ������ | �� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ���ɫҺ�� |
| �ܶ�/��g•cm-3�� | 0.79 | 1.44 | 3.1 |
| �е�/�� | 78.5 | 38.4 | 59 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| ʵ�� ��� | NaOH��Һ��Ũ�ȣ�mol/L�� | �ζ����ʱ��NaOH��Һ����������mL�� | ����HCl��Һ�������mL�� |
| 1 | 0.10 | 22.62 | 20.00 |
| 2 | 0.10 | 22.72 | 20.00 |
| 3 | 0.10 | 22.80 | 20.00 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij����С�����о��������������������£��ı���ʼ���������ʵ����Ժϳ�������������Ӧ 4NO2��g��+O2��g��?2N2O5��g����H��0��Ӱ�죮
ij����С�����о��������������������£��ı���ʼ���������ʵ����Ժϳ�������������Ӧ 4NO2��g��+O2��g��?2N2O5��g����H��0��Ӱ�죮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£���22.4LHCl����1Lˮ�������1mol•L-l��ϡ���� | |
| B�� | ��100mL18mol•L-1��ŨH2SO4��100mLˮ��ϣ������9moI•L-l��H2SO4��Һ | |
| C�� | ��4.0gNaOH����100mL����ƿ�У���ˮ���̶��ߣ����1mol•L-1��NaOH��Һ | |
| D�� | ��0.1molNaCl���100mL��Һ������ȡ��10mL����ȡ����Һ�����ʵ���Ũ��Ϊ1mol•L-1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com