
 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2012?����ģ�⣩Ϊ�ⶨNa2CO3��Na2SO3������и���ֵĺ������������ʵ�鷽����
��2012?����ģ�⣩Ϊ�ⶨNa2CO3��Na2SO3������и���ֵĺ������������ʵ�鷽�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
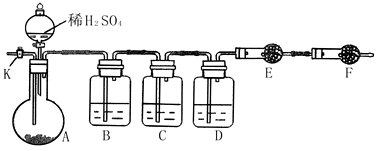
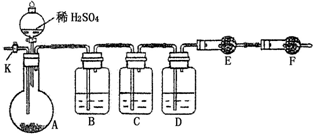
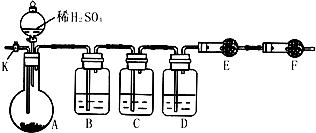
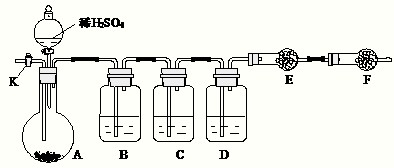
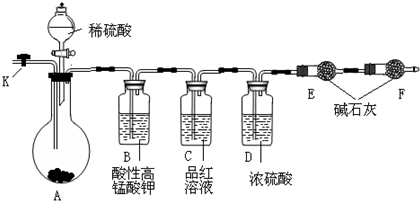
��14�֣�Ϊ�ⶨNa2CO3��Na2SO3������и���ֵĺ�����ȡ��Ʒ23.2g����ͼ��ʾװ�ý���ʵ�飺������̨�����е�����δ��ͼ�л�����
������F�������� ��
����֪����C��װ��Ʒ����Һ���������� �������������Һ��������Na2CO3�����IJ��������ʵ��ֵƫ�ͣ������� ��
��ʵ�����б������³����Լ���a.Ũ���� b.Ʒ����Һ c.���Ը��������Һ d.����������Һ e.��ˮ����ͭ f.��ʯ�� g.���������� h.��ˮ�Ȼ��ƣ��뽫����������Ӧʢ�ŵ��Լ����������Ӧ�ո�B�� ��D�� ��E�� ��
ʵ������У�������A�ڵĹ��巴Ӧ��ȫ�������K����A��ͨ������Ŀ�������������Ŀ����ʹA��B��C��D�������в�����CO2����E��������ա���ͨ����Ӧ�Ⱦ���___________�Լ��������������Լ�����ţ������������������Na2SO3�����IJ��������ʵ��ֵ_________���ƫ�ߡ�����ƫ�͡���ûӰ�족����
��������E���������ʱ����4.4g����Na2CO3��Na2SO3�����ʵ���֮��Ϊ_______������4.4g��ֵ��õķ�����_______________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com