
ijѧ����0.1000mol/L KOH��Һ�ζ�δ֪���Ե�������Һ��������ɷֽ�Ϊ���¼�����
��A����ȡ20.00mL�����������Һע��ྻ����ƿ��������2-3�η�̪
��B���ñ���Һ��ϴ�ζ���2-3��
��C����ʢ�б���Һ�ļ�ʽ�ζ��̶ܹ��ã�����Һ��ʹ�ζ��ܼ��������Һ
��D��ȡ��KOH��Һע���ʽ�ζ�����0�̶�����2-3cm
��E������Һ����0��0�̶����£����¶���
��F������ƿ���ڵζ��ܵ����棬�ñ�KOH��Һ�ζ����յ㣬���µζ���Һ��Ŀ̶�
���������գ�
��1����ȷ������˳���ǣ��������ĸ��д��______________________.
��2��������B��������Ŀ����___________________________________��
��3��������A������֮ǰ�������ô���Һ��ϴ��ƿ����Բⶨ�����Ӱ���ǣ���ƫ��ƫС�����䣬��ͬ��_________________________��
��4��ʵ���������ֿ���____________������������λ�����۾�ע��____________________��ֱ���ζ��յ㡣�жϵ����յ��������________________________________________��
��5������ȡһ������KOH���壨������NaOH�����Ʊ���Һ�������ζ��������ᣬ��Բⶨ�����Ӱ����___________________________________________________-��
��6���ζ������������ӹ۲�ζ�����Һ��̶ȣ���Եζ������Ӱ����______________��
��1��BDCEAF
��2����ֹ����Һϡ��
��3��ƫ��
��4���ζ��ܻ�������ƿ����Һ����ɫ�仯����ƿ����Һ����ɫ����ɫ��dz���ұ���30���ڲ���ɫ��
��5��ƫС ��6��ƫ��
��������
�����������1���к͵ζ����ռ�©��ϴ�ӡ���ϴ��װҺ���ζ���˳���������˸�������к͵ζ�ԭ����֪����ȷ�IJ���˳����BDCEAF��
��2���ζ����ñ�Һ��ϴ��Ŀ���Ƿ�ֹ����Һϡ�ͣ�����ʵ����
��3����ƿ����ô���Һ��ϴ������ƿ�����ʵ����ʵ��������ӣ������������������Һ��������ӣ��Ӷ��ɵ��²�ý��ƫ�ߡ�
��4��ʵ���������ֿ��Ƶζ��ܻ������۾�ע����ƿ����Һ����ɫ�仯��ֱ���ζ��յ㡣����ѡ���˷�̪��ָʾ���������жϵ����յ����������ƿ����Һ����ɫ����ɫ��dz���ұ���30���ڲ���ɫ��
��5�������������Ƶ�Ħ������С���������صģ��������Ƶı�Һ��OH����Ũ��ƫ����������Һ��������٣��ⶨ���ƫС��
��6���ζ��ܵĿ̶������϶������������Եζ������������ӹ۲�ζ�����Һ��̶ȣ����ʱ����ƫ���������ĵ���Һ������ӣ���ⶨ���ƫ��
���㣺��������к͵ζ�ԭ��������ע�������Լ���������
���������⿼������к͵ζ�ʵ�飬��Ŀ�ѶȲ�����ʱע�����ʵ���ԭ�������衢�����Լ�ע���������ʵ�����������Ȼ��������ü��ɡ�������ѵ���Ȼ������������������������Ϊ����C�⣽C��V��/V������C�ꡢV����Ϊ��ֲ������C��Ĵ�Сȡ����V��Ĵ�С����V�꣺ƫ���ƫС����C��ƫ���ƫС���ݴ˿��Խ����й��жϡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�ijѧ����0.1000 mol��L��1������������Һ�ζ�δ֪Ũ�ȵ�������Һ��������ɷֽ�Ϊ���¼�����
A��ȡ20.00 mL����������Һע��ྻ����ƿ�У�������2��3�η�̪��Һ
B���ñ�����������Һ��ϴ�ζ���2��3��
C����ʢ�б�����������Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ
D��ȡ������������Һע���ʽ�ζ�������0���̶�����2��3mL��
E������Һ������0����0���̶����£����¶�����Ϊ3.10mL
F������ƿ���ڵζ������棬�ñ�NaOH��Һ�ζ����յ㲢���µζ��ܵĶ�����
�ش��������⣺
��1����ȷ�IJ���˳���ǣ�B�� ��F������ţ���
��2������B���������Ŀ���� ��
��3������A�������֮ǰ�������ô�����Һ��ϴ��ƿ����Եζ������Ӱ��
�� ���ƫ��ƫС������Ӱ�족����
��4���жϵ���ζ��յ��ʵ�������� ��
��5�����ζ�����ʱ���ζ���Һ����ͼ��ʾ�����յ����Ϊ mL��
��6�����ݶ���������ô���Һ��Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�ijѧ����0.1000 mol��L��1������������Һ�ζ�δ֪Ũ�ȵ�������Һ��������ɷֽ�Ϊ���¼�����
A��ȡ20.00 mL����������Һע��ྻ����ƿ�У�������2��3�η�̪��Һ
B���ñ�����������Һ��ϴ�ζ���2��3��
C����ʢ�б�����������Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ
D��ȡ������������Һע���ʽ�ζ�������0���̶�����2��3mL��
E������Һ������0����0���̶����£����¶�����Ϊ3.10mL
F������ƿ���ڵζ������棬�ñ�NaOH��Һ�ζ����յ㲢���µζ��ܵĶ�����
�ش��������⣺
��1����ȷ�IJ���˳���ǣ�B�� ��F������ţ���
��2������B���������Ŀ���� ��
��3������A�������֮ǰ�������ô�����Һ��ϴ��ƿ����Եζ������Ӱ��
�� ���ƫ��ƫС������Ӱ�족����
��4���жϵ���ζ��յ��ʵ�������� ��
��5�����ζ�����ʱ���ζ���Һ����ͼ��ʾ�����յ����Ϊ mL��
��6�����ݶ���������ô���Һ��Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ��Ǩ�������غ�����ѧ�߶���ѧ�����п���ѧ������������ ���ͣ�ʵ����
ijѧ����0.1000 mol��L��1������������Һ�ζ�δ֪Ũ�ȵ�������Һ��������ɷֽ�Ϊ���¼�����
A��ȡ20.00 mL����������Һע��ྻ����ƿ�У�������2��3�η�̪��Һ
B���ñ�����������Һ��ϴ�ζ���2��3��
C����ʢ�б�����������Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ
D��ȡ������������Һע���ʽ�ζ�������0���̶�����2��3mL��
E������Һ������0����0���̶����£����¶�����Ϊ3.10mL
F������ƿ���ڵζ��ܵ����棬�ñ�NaOH��Һ�ζ����յ㲢���µζ��ܵĶ������ش��������⣺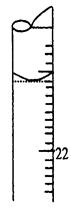
��1����ȷ�IJ���˳���ǣ�B�� ��F������ţ���
��2������B���������Ŀ���� ��
��3������A�������֮ǰ�������ô�����Һ��ϴ��ƿ����Եζ������Ӱ��
�� ���ƫ��ƫС������Ӱ�족����
��4���жϵ���ζ��յ��ʵ�������� ��
��5�����ζ�����ʱ���ζ���Һ����ͼ��ʾ�����յ����Ϊ mL��
��6�����ݶ���������ô���Һ��Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�����и߶����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��12�֣�ijѧ����0.1000 mol��L��1������������Һ�ζ�δ֪Ũ�ȵ�������Һ��������ɷֽ�Ϊ���¼�����
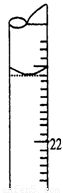
A��ȡ20.00 mL����������Һע��ྻ����ƿ�У�������2��3�η�̪��Һ
B���ñ�����������Һ��ϴ�ζ���2��3��
C����ʢ�б�����������Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ
D��ȡ������������Һע���ʽ�ζ�������0���̶�����2��3mL��
E������Һ������0����0���̶����£����¶�����Ϊ3.10mL
F������ƿ���ڵζ������棬�ñ�NaOH��Һ�ζ����յ㲢���µζ��ܵĶ�����
�ش��������⣺
��1����ȷ�IJ���˳���ǣ�B�� ��F������ţ���
��2������B���������Ŀ���� ��
��3������A�������֮ǰ�������ô�����Һ��ϴ��ƿ����Եζ������Ӱ��
�� ���ƫ��ƫС������Ӱ�족����
��4���жϵ���ζ��յ��ʵ�������� ��
��5�����ζ�����ʱ���ζ���Һ����ͼ��ʾ�����յ����Ϊ mL��
��6�����ݶ���������ô���Һ��Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ�����и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��12�֣�ijѧ����0.1000 mol��L��1������������Һ�ζ�δ֪Ũ�ȵ�������Һ��������ɷֽ�Ϊ���¼�����
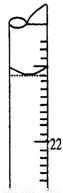
A��ȡ20.00 mL����������Һע��ྻ����ƿ�У�������2��3�η�̪��Һ
B���ñ�����������Һ��ϴ�ζ���2��3��
C����ʢ�б�����������Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ
D��ȡ������������Һע���ʽ�ζ�������0���̶�����2��3mL��
E������Һ������0����0���̶����£����¶�����Ϊ3.10mL
F������ƿ���ڵζ������棬�ñ�NaOH��Һ�ζ����յ㲢���µζ��ܵĶ�����
�ش��������⣺
��1����ȷ�IJ���˳���ǣ�B�� ��F������ţ���
��2������B���������Ŀ���� ��
��3������A�������֮ǰ�������ô�����Һ��ϴ��ƿ����Եζ������Ӱ��
�� ���ƫ��ƫС������Ӱ�족����
��4���жϵ���ζ��յ��ʵ�������� ��
��5�����ζ�����ʱ���ζ���Һ����ͼ��ʾ�����յ����Ϊ mL��
��6�����ݶ���������ô���Һ��Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com