
| A£® | °×É«¹ĢĢåŅ»¶ØÖ»ŗ¬ÓŠĮņĖįļ§ | |
| B£® | ČōĒāŃõ»ÆÄĘČÜŅŗ×ćĮ棬ŌņÉś³É°±ĘųµÄĢå»żÓ¦ĪŖ6.72L£Ø±źæö£© | |
| C£® | “ÓÉĻŹöŹż¾ŻÄÜĒóĖć³ö°×É«¹ĢĢåÖŠ£ØNH4£©2SO4”¢NH4HSO4µÄĪļÖŹµÄĮæÖ®±ČĪŖ1£ŗ2 | |
| D£® | ½«Ä³°×É«¹ĢĢå¼ÓČČ·Ö½ā£¬Čō²śÉśµÄĘųĢå²»ÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£¬ŌņøĆ¹ĢĢåÖŠŅ»¶Ø²»ŗ¬ļ§ŃĪ |
·ÖĪö A£®Éč²ĪÓė·“Ó¦µÄ£ØNH4£©2SO4ŗĶNH4HSO4£¬·Ö±šĪŖxmol£¬ymol£¬
£ØNH4£©2SO4+2NaOH=2NH3”ü+Na2SO4+2H2O
1 2 2
x 2x 2x
NH4HSO4+2NaOH=NH3ӟ+Na2SO4+2H2O
1 2 1
y 2y y
n£ØNH3£©=$\frac{2.24L}{22.4L/mol}$=0.1mol£¬Ōņ2x+y=0.1¢Ś£¬n£ØNaOH£©=2x+2y=0.05L”Į4mol/L¢Ś£¬ĮŖĮ¢¢Ł¢Ś£¬¾Ż“Ė½ųŠŠ·ÖĪö¹ĢĢåµÄ³É·Ö£»
B£®ÓÉAµĆn£ØNH4HSO4£©=0.1mol£¬m£Ø£ØNH4£©2SO4£©=24.7g-0.1mol”Į115g/mol=13.2g£¬¹Źn£Ø£ØNH4£©2SO4£©=$\frac{13.2g}{132g/mol}$=0.1mol£¬²¢¾ŻµŖŌŖĖŲŹŲŗćn£ØNH3£©=2n£Ø£ØNH4£©2SO4£©+n£ØNH4HSO4£©=2”Į0.1mol+0.1mol=0.3mol£¬²¢¾ŻV=nVm½ųŠŠ¼ĘĖć£»
C£®½įŗĻABÖŠŹż¾Ż·ÖĪö£»
D£®¾Żļ§ŃĪ¼ÓČČĢõ¼žĻĀæÉÄÜÉś³ÉµŖĘų·ÖĪö£®
½ā“š ½ā£ŗA£®Éč²ĪÓė·“Ó¦µÄ£ØNH4£©2SO4ŗĶNH4HSO4£¬·Ö±šĪŖxmol£¬ymol£¬
£ØNH4£©2SO4+2NaOH=2NH3”ü+Na2SO4+2H2O
1 2 2
x 2x 2x
NH4HSO4+2NaOH=NH3ӟ+Na2SO4+2H2O
1 2 1
y 2y y
n£ØNH3£©=$\frac{2.24L}{22.4L/mol}$=0.1mol£¬Ōņ2x+y=0.1¢Ś£¬n£ØNaOH£©=2x+2y=0.05L”Į4mol/L¢Ś£¬ĮŖĮ¢¢Ł¢Ś£¬½āµĆx=0£¬y=0.1£¬¹Ź¹ĢĢåÖŠæĻ¶ØÓŠNH4HSO4£¬¹Źm£ØNH4HSO4£©=0.1mol”Į115g/mol=11.5g£¬11.5g£¼24.70g£¬¹Źŗ¬ £ØNH4£©2SO4ŗĶNH4HSO4£¬¹ŹA“ķĪó£»
B£®ÓÉAµĆn£ØNH4HSO4£©=0.1mol£¬m£Ø£ØNH4£©2SO4£©=24.7g-0.1mol”Į115g/mol=13.2g£¬¹Źn£Ø£ØNH4£©2SO4£©=$\frac{13.2g}{132g/mol}$=0.1mol£¬²¢¾ŻµŖŌŖĖŲŹŲŗćn£ØNH3£©=2n£Ø£ØNH4£©2SO4£©+n£ØNH4HSO4£©=2”Į0.1mol+0.1mol=0.3mol£¬²¢¾ŻV=nVm=0.3mol”Į22.4L/mol=6.72L£¬¹ŹBÕżČ·£»
C£®ÓÉA£¬BµĆn£ØNH4HSO4£©=0.1mol£¬n£Ø£ØNH4£©2SO4£©=$\frac{13.2g}{132g/mol}$=0.1mol£¬¹Ź£ØNH4£©2SO4”¢NH4HSO4µÄĪļÖŹµÄĮæÖ®±ČĪŖ1£ŗ1£¬¹ŹC“ķĪó£»
D£®Čōļ§ŃĪ¼ÓČČĢõ¼žĻĀÉś³ÉµŖĘų£¬ŌņµŖĘų²»ÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£¬¹ŹD“ķĪó£»
¹ŹŃ”B£®
µćĘĄ ±¾Ģāæ¼²éĮĖ»ģŗĻĪļ×é³ÉµÄĶʶĻ£¬Ķź³É“ĖĢā£¬æÉŅŌ½įŗĻĢāøÉĢį¹©µÄŠÅĻ¢½ųŠŠ·ÖĪö£¬²¢½įŗĻŌ×ÓŹŲŗć½ųŠŠ¼ĘĖć£¬ÄѶČÖŠµČ£®


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ņ»øö¼×Č©·Ö×ÓÓÉ1øöĢ¼Ō×Ó”¢2øöĒāŌ×Ó”¢1øöŃõŌ×Ó¹¹³ÉµÄ | |
| B£® | ¼×Č©ÖŠĢ¼”¢Ēā”¢ŃõČżÖÖŌŖĖŲµÄÖŹĮæ±ČĪŖ1£ŗ2£ŗ1 | |
| C£® | ¼×Č©ÓÉĢ¼”¢Ēā”¢ŃõČżÖÖŌŖĖŲ×é³ÉµÄ | |
| D£® | ¼×Č©ÖŠŃõŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ53.3% |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ŹµŃé²Ł×÷ŗĶĻÖĻó | ½įĀŪ |
| A | ȔɣĮæ“ż¼ģŃéČÜŅŗ£¬ĻņĘäÖŠ¼ÓČėÉŁĮæŠĀÖĘĀČĖ®£¬ŌŚµĪ¼ÓKSCNČÜŅŗ£¬ČÜŅŗ³ŹŃŖŗģÉ« | “ż²āŅŗÖŠŗ¬ÓŠFe3+ |
| B | ŹŅĪĀĻĀ£¬ĻņÅØ¶Č¾łĪŖ0.1mol•L-1µÄBaCl2ŗĶCaCl2»ģŗĻČÜŅŗÖŠµĪ¼ÓNa2SO4ČÜŅŗ£¬³öĻÖ°×É«³Įµķ£® | Ksp£ØBa2SO4£©£¼Ksp£ØCa2SO4£© |
| C | ŹŅĪĀĻĀ£¬ĻņFeCl3ČÜŅŗÖŠµĪ¼ÓÉŁĮæKIČÜŅŗ£¬ŌŁµĪ¼Ó¼øµĪµķ·ŪČÜŅŗ£¬ČÜŅŗ±äĄ¶É« | Fe3+µÄŃõ»ÆŠŌ±ČI2µÄĒæ |
| D | ŹŅĪĀĻĀ£¬ÓĆPHŹŌÖ½²āµĆ£ŗ0.1mol•L-1 Na2SO3ČÜŅŗµÄPHŌ¼ĪŖ10£»0.1mol•L-1 NaHSO3ČÜŅŗµÄPHŌ¼ĪŖ5 | HSO3-½įŗĻH+µÄÄÜĮ¦±ČSO32-µÄĒæ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µāĖ®ÖŠ¼ÓČėÉŁĮæĘūÓĶÕńµ“£¬¾²ÖĆŗóÉĻ²ćŃÕÉ«±äĒ³£¬ĻĀ²ćŃÕÉ«±äĪŖ×ĻŗģÉ« | |
| B£® | ŗģČȵÄĶĖæŌŚĀČĘųÖŠČ¼ÉÕ£¬²śÉśĮĖ×Ų»ĘÉ«µÄŃĢĪķ | |
| C£® | Ā±ĖŲµ„ÖŹ£ØX2£©ÓėĖ®·“Ó¦¾łæÉÓĆX2+H2O=HX+HXO±ķŹ¾ | |
| D£® | äå»ÆÄĘČÜŅŗÖŠ¼ÓČėÉŁĮæŠĀÖʵÄĀČĖ®Õńµ“£¬ŌŁ¼ÓČėÉŁĮæĖÄĀČ»ÆĢ¼Õńµ“£¬¾²ÖĆŗóÉĻ²ćŃÕÉ«±äĒ³£¬ĻĀ²ćŃÕÉ«±äĪŖ³ČŗģÉ« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
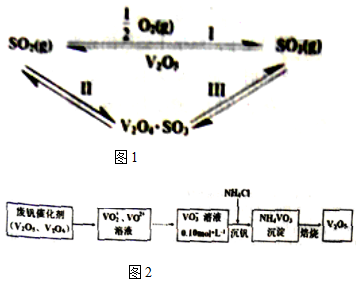
| »Æѧ¼ü | S=O£ØSO2£© | S=O£ØSO3£© | O=O£ØO2£© |
| ÄÜĮæ/kJ | 535 | 472 | 496 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ³żČ„»ģŌŚCu·ŪÖŠµÄÉŁĮæMg·ŪŗĶAl·Ū£¬¼ÓĻ”ŃĪĖįŗó¹żĀĖ | |
| B£® | ·ÖĄėĘūÓĶŗĶĆŗÓĶ£¬æÉÓĆŻĶČ”µÄ·½·Ø | |
| C£® | ·ÖĄėĻõĖį¼ŲŗĶĀČ»ÆÄĘ¹ĢĢåµÄ»ģŗĻĪļ£¬æÉÓĆČܽā”¢¹żĀĖµÄ·½·Ø | |
| D£® | ½«ŃõĘųŗĶĒāĘųµÄ»ģŗĻĘųĢåĶعż×ĘČȵÄŃõ»ÆĶ£¬ŅŌ³żČ„ĘäÖŠµÄĒāĘų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
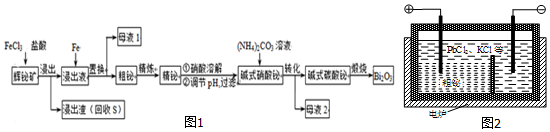
 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com