
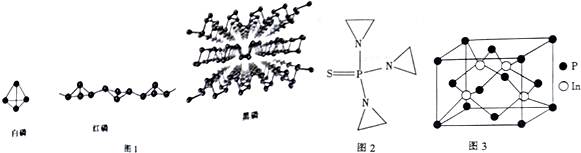
���� ��1������ԭ�Ӻ���ÿ�����ӵ��˶�״̬���ɶ��״̬���������������������������������ȣ�ͬһ����û���������ӵĸ���������ȫ��ͬ���ʶ�ԭ�Ӻ���ÿ�����ӵ��˶�״̬���Dz�ͬ�ģ�Ȼ����ݺ����������������
��2���ٺ��ṹ��ʯī���ƣ������ܵ��磻
��P4����Ϊ�������幹�ͣ�P4������Nԭ���γ�3���Ҽ�������1�Թ¶Ե��ӣ��ӻ������ĿΪ4��������������ԭ�����
�ۺ����۷е�ϵͣ�ӦΪ���Ӿ��壻
��3��ͬ��Ԫ�غ����ᣬ��Ԫ�ػ��ϼ�Խ�ߣ�������Խǿ�����ǻ���Խ�࣬����Խǿ��PO43-���ӵ����幹�Ϳ��ɼ۲���ӶԻ�������ȷ����
��4�������߷����У�Nԭ�Ӻ���3�� �� ����һ���µ��Ӷԣ�����ʽ��ʡ�Ե���C��Hԭ�ӣ�
��5�����ݾ�̯�����㾧����ԭ�Ӹ����������ܶ�$��=\frac{m}{V}$���㣮
��� �⣺P����15��Ԫ�أ���������˶�״̬����15�֣���۵����Ų�ʽΪ3s23p3���ʴ�Ϊ��15��3s23p3��
��2�����ṹ��ʯī���ƣ������ܵ��磬�ʴ�Ϊ�����ף�
��P4����Ϊ�������幹�ͣ�P4������Nԭ���γ�3���Ҽ�������1�Թ¶Ե��ӣ��ӻ������ĿΪ4��Pԭ�Ӳ�ȡsp3�ӻ���
��������ԭ����ָ���ڼ��Է��Ӽ�ĵ������ã�ʹ�ü��Է�����ɵ����������ڼ��Է�����ɵ��ܼ��������ڷǼ��Է�����ɵ��ܼ����Ǽ��Է�����ɵ����������ڷǼ��Է�����ɵ��ܼ��������ڼ��Է�����ɵ��ܼ���P4��CS2�ǷǼ��Է��ӣ������������ܵ�ԭ����P4������ CS2��
�ʴ�Ϊ��4��P4��CS2�ǷǼ��Է��ӣ������������ܵ�ԭ����P4������ CS2��
�ۺ����۵�Ϊ72�棬�е�Ϊ350�棬�۷е�ϵͣ��������ڷ��Ӿ��壬�ʴ�Ϊ�����ӣ�
��3��H3PO2�������ᣩ��H3PO3�������ᣩ��H3PO4��PԪ�ػ��ϼ�����Ϊ+1��+3��+5��Ԫ�ػ��ϼ�Խ�ߣ�������Խǿ�����ǻ���Խ�࣬����Խǿ��������H3PO4��H3PO3��H3PO2��PO43-��Pԭ�ӵļ۲���Ӷ�=4+$\frac{1}{2}$��5+3-4��2��=4���Ҳ����µ��Ӷԣ�������ռ乹���������壬��PO43-�ռ乹����ͬ�ķ��Ӻ������ӷֱ���CCl4����SiCl4�ȣ���SO42-����ClO4-��SiO42-�ȣ���
�ʴ�Ϊ��H3PO4��H3PO3��H3PO2��CCl4����SiCl4�ȣ���SO42-����ClO4-��SiO42-�ȣ���
��4�������߷����У�Nԭ�Ӻ���3�� �� ����һ���µ��Ӷԣ����Բ�ȡsp3�ӻ������ϵ�Cԭ�Ӻ���4�� �� ����һ�������߷����к���25���� ��������1mol�����߷����� �� ��Ϊ25mol���ʴ�Ϊ��sp3��25NA��
��5��������InP����Pԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��Inԭ����ĿΪ4����������m=$\frac{��115+31��g/mol��4}{6.02��1{0}^{23}mo{l}^{-1}}$���������V=a3���ܶ�$��=\frac{m}{V}$=4.8g•cm-3���ʴ�Ϊ��4.8��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų����ӻ���ʽ�жϡ���������ȣ�����ؼ��ǽ�ϻ�ѧʽ����̯�����㾧����ԭ����Ŀ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 HN03�� HN02�ǵ���������Ҫ�����ᣮHN02�ܱ����������������������ء��ظ���صȣ�������������������Ҳ�ܱ��������ӡ������ӻ�ԭ������������ AgN02 ����һ��������ˮ����������Ļ�����ش��������⣺
HN03�� HN02�ǵ���������Ҫ�����ᣮHN02�ܱ����������������������ء��ظ���صȣ�������������������Ҳ�ܱ��������ӡ������ӻ�ԭ������������ AgN02 ����һ��������ˮ����������Ļ�����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 AIN����Ҫ�İ뵼����ϣ�Ga���أ���P��As���飩�����γɻ�����뵼����ϵ���ҪԪ�أ�
AIN����Ҫ�İ뵼����ϣ�Ga���أ���P��As���飩�����γɻ�����뵼����ϵ���ҪԪ�أ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| W | ||||
| X | Y | Z |
| A�� | Ԫ��X�ĵ�������ǿ�ᡢǿ�Ӧ | |
| B�� | �������ӵĻ�ԭ�ԣ�W��Z | |
| C�� | ��̬�⻯����ȶ��ԣ�W��Y | |
| D�� | Ԫ��W��X����Ԫ�ؿ��γɻ�����Na3XW6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ԭ��N���϶���1��s���ӣ�һ��ԭ����d���ӣ���һ��ԭ����d���� | |
| B�� | ��ԭ�������ȫ������s���� | |
| C�� | ���������Ų�Ϊ2s22p5��ԭ�Ӻ����������Ų�Ϊ2s22p6������ | |
| D�� | ԭ�Ӻ���M���ϵ�s��p�ܼ����������ӣ���d�ܼ���û�е��ӵ�����ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͨ����С�ڵ�����ʾ���ӵĶ��٣��ڵ��ܶȴ�����Ŀ�� | |
| B�� | �ڵ��ܶȴ�С����ʾ��λ����ڵ��ӳ��ֵĻ������ | |
| C�� | ͨ����С�ڵ�����ʾ�����ƺ�������Բ���˶� | |
| D�� | ������ͼ�Ƕ��˶������Ե����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��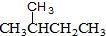 ��CH4��CH3CH3
��CH4��CH3CH3�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
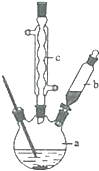 ������ͭ�Ǻϳ��������������в���--��������ͭ����Ҫ
������ͭ�Ǻϳ��������������в���--��������ͭ����Ҫ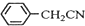 +H2O+H2SO4$\stackrel{100-130��}{��}$
+H2O+H2SO4$\stackrel{100-130��}{��}$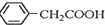 +NH4HSO4
+NH4HSO4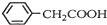 +Cu��OH��2����
+Cu��OH��2����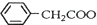 ��2Cu+H2O
��2Cu+H2O�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com