
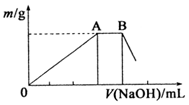
 �����仯����������������ȷ����й㷺��Ӧ�ã���ش��������⣺
�����仯����������������ȷ����й㷺��Ӧ�ã���ش��������⣺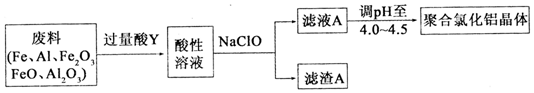
���� ��1������ͬ����Ԫ��ԭ������Խ������Խǿ�����Ӧ���μ���Խǿ��
��2���٣�NH4��Al��SO4��2��Һ�������ӡ�笠����ӷ���ˮ��ʹ��Һ�����ԣ�������ˮ��������ǿ���ݴ˷�����
����NH4��Al��SO4��2��Һ�еμ�1mol•L-1 NaOH��Һ��OA��Ϊ�����������������ӷ�Ӧ������������������AB��Ϊ������������笠����ӵķ�Ӧ��
��3��������������������Ϊ��ʧȥ���ӷ���������Ӧ���ݴ˷�����
��4���ٸ��ݻ����ﻯ�ϼ۴�����Ϊ0�ɵã�
�������̿�֪��������м��Ὣ���������ܽ⣻
�۷�����ӦFe3++3OH-=Fe��OH��3����Al3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2O �ۣ�OH-����ʱ��Al3+��Fe3+���ɳ�������OH-����ʱ��Al��OH��3�ܽ⣻���ü��ķ������н��
��� �⣺��1��ͬ����Ԫ��ԭ������Խ������Խǿ�����Ӧ������Һ����Խǿ���ء�������λ��ͬһ���壬�������أ������𣬹ʼ���Na[Ga��OH��4]��Na[A1��OH��4]��Na[B��OH��4]��
�ʴ�Ϊ���ڢ٢ۣ�
��2���٣�NH4��Al��SO4��2��Һ�������ӡ�笠����ӷ���ˮ��ʹ��Һ�����ԣ�������ˮ��������ǿ��������Ũ����С�����˳��Ϊ��c��OH-����c��H+����c��Al3+����c��NH4+����c��SO42-����
�ʴ�Ϊ��c��OH-����c��H+����c��Al3+����c��NH4+����c��SO42-����
����NH4��Al��SO4��2��Һ�еμ�1mol•L-1 NaOH��Һ��OA��Ϊ�����������������ӷ�Ӧ������������������AB��Ϊ������������笠����ӵķ�Ӧ������ʽΪ��NH4++OH-=NH3•H2O��
�ʴ�Ϊ��NH4++OH-=NH3•H2O��
��3�������������������£�����Һ�壨���л������ӡ�A12C17-��A1C14-��ɣ���Ϊ�������Һ������ʧȥ���ӷ���������Ӧ���������ĵ缫��ӦʽΪAl-3e-+7AlCl4-=4Al2Cl7-��
�ʴ�Ϊ��Al-3e-+7AlCl4-=4Al2Cl7-��
��4����[A12��OH��aC1b•nH2O]m��������+3�ۣ�������-1�ۣ�����������-1�ۣ������ﻯ�ϼ۴�����Ϊ0����a+b=6��
�ʴ�Ϊ��a+b=6��
�������̿�֪��������м��Ὣ���������ܽ⣬�����Һ�м���������Ƶõ���������Һ�ϳɲ�Ʒ�������Ҳ����������˵���������ƽ�����Һ��������������������pH����������Ϊ���������ᣬ�ܽ�Ľ�������һ����Fe2+��Al3+��
�ʴ�Ϊ��Fe2+��Al3+��
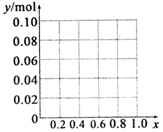
��Al3+��Fe3+�����ʵ���֮��Ϊ0.10mol��n��NaOH��=2mol/L��0.17L=0.34mol��
����Һ�в�����������ʱ����x=0ʱ����Һ�д��ڵij���ΪFe��OH��3����ԭ���غ�ó��������ʵ���=n��Fe��OH��3��=n��Fe3+��=0.10mol��
��x=0.4ʱ��n��Al3+��=0.04mol��n��Fe3+��=0.06mol����������ȫ������Ҫn��NaOH��=3n��Fe3+��=0.18mol����������ȫ������Ҫn��NaOH��=3n��Al3+��=0.12mol��ʵ����n��NaOH�����������Ӻ���������ȫ������Ҫ���������Ƶ����ʵ�����������������Ҫ�ܽ⣬��������������ȫ�ܽ⣬����Ҫn��NaOH��=n��Al3+��=0.04mol��������������ǡ����ȫ�ܽ⣬�����ɳ��������ʵ��������������������ʵ�����Ϊ0.06mol��
��x=0.6ʱ��0.06mol��Al3+ת��Ϊ0.06molAl��OH��3��Ҫn��NaOH��=0.18mol��n��Fe3+��=0.04mol����������ȫ������Ҫn��NaOH��=3n��Fe3+��=0.12mol����ʣ��0.04mol�������ƺ�0.04mol����������Ӧ����ƫ�����ƣ���ʣ��������������0.02mol�����Գ��������ʵ���=0.04mol-0.02mol=0.06mol��
��0��x��0.6��ͼ��Ϊ�� ��
��
�ʴ�Ϊ�� ��
��
���� ��������Ϊ���壬�����˻����ļ��㡢��Һ������Ũ�ȵıȽϡ��绯ѧ��֪ʶ�㣬��������������ʵ���Ϣ��ͼ���ȡ�����Ŀ��飬���������Ӻ�����������Һ��Ӧ�ص���ü��ķ������з������ٽ��ԭ���غ���н����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 ��������{[CH3CH��OH��COO]2Fe}��һ�ֺܺõ�ʳƷ��ǿ������������ˮ������Ч���������ã�����������FeCO3��Ӧ�Ƶã�������һ���Ʊ�����������ʵ�鷽����
��������{[CH3CH��OH��COO]2Fe}��һ�ֺܺõ�ʳƷ��ǿ������������ˮ������Ч���������ã�����������FeCO3��Ӧ�Ƶã�������һ���Ʊ�����������ʵ�鷽�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ���¶Ⱥ�ѹǿ�£�������̬��������Ĵ�С���ɹ�������ķ��Ӵ�С���� | |
| B�� | ��ͬ�����壬�������ͬ�������������ķ���������ͬ | |
| C�� | �����Ħ�������ָ1mol�κ�������ռ���������22.4L | |
| D�� | ���º�ѹ�����£���Ӧǰ����������֮�ȵ�����������ʵ���֮�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
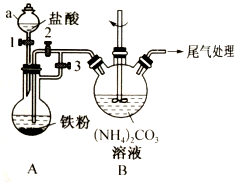
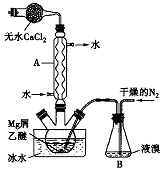 ��ˮMgBr2������������ʵ���Ҳ���þм��Һ��Ϊԭ���Ʊ���ˮMgBr2��װ����ͼ��ʾ���г�������ȥ������Ҫ�������£�
��ˮMgBr2������������ʵ���Ҳ���þм��Һ��Ϊԭ���Ʊ���ˮMgBr2��װ����ͼ��ʾ���г�������ȥ������Ҫ�������£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NO | B�� | Ca��ClO��2 | C�� | NaCl | D�� | NaHCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | С�Թ� | B�� | ����ƿ | C�� | ��Ͳ | D�� | ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
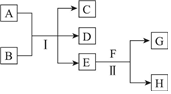 A��HΪ��ѧ��ѧ�м��ֳ������ʣ�����֮���ת����ϵ��ͼ��ʾ�������£�A��G��Ϊ�������壬ͬ��C�������������EΪ��ɫ����ζ��Һ�壬FΪ����ɫ��ĩ���ش��������⣺
A��HΪ��ѧ��ѧ�м��ֳ������ʣ�����֮���ת����ϵ��ͼ��ʾ�������£�A��G��Ϊ�������壬ͬ��C�������������EΪ��ɫ����ζ��Һ�壬FΪ����ɫ��ĩ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NO | B�� | O2 | C�� | NH3 | D�� | NO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com