
����Ŀ��2-����-l��3-�������ɼ䱽�����Ȼǻ�������������ȥ��������ɡ�ԭ�����£�
�������ʵ�����������£�
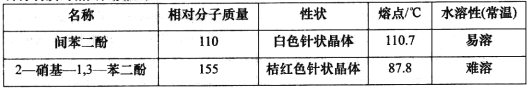
�Ʊ��������£�
��һ�����ǻ�����ȡ71.5g�䱽���ӣ���ɷ�ĩ������ƿ�У�������������Ũ���Ტ���Ͻ��裬�����¶���һ����Χ��15min(��ͼ1)��
�ڶ��������������ǻ���Ӧ��������ƿ������ˮ�У������ȴ����롰���ᡱ�������¶ȼ�������l5min��
������������������Ӧ�����ϡ��Һת�Ƶ�Բ����ƿB�У�Ȼ����ͼ2��ʾװ�ý���ˮ��������

��ش��������⣺
��1��ʵ�����а��ʱ�������ɷ�ĩ��Ҫ�IJ���������________��
��2���ǻ������п����¶�����ʵķ�ΧΪ(����ĸ)________��
a��30����60�� b��60����65�� c��65����70�� d��70����100��
��3��������������ȡ�����ᡱ�ľ��������____________��
��4��ͼ2�У���ƿA�г�����������ѹ���ã����ܷ�ֹװ����ѹǿ���������¹ʣ����ܷ�ֹ___________��ֱ��������C�е�������_________����Ӧһ��ʱ���ֹͣ����ʱ�IJ�����____________(���й������;ƾ��ƵIJ���)��
��5��ˮ���������Ƿ�����ᴿ�л���ķ���֮һ�����ᴿ���ʱ���߱���������ȷ��___________��
a�����ܻ�������ˮ������������ b���ڷ�������ˮ��������ѧ��Ӧ
c������һ���Ļӷ��� d�����нϵ͵��۵�
��6����ʵ�����ջ��12.0g�ۺ�ɫ���壬��2-����-1��3-�����ӵIJ���ԼΪ_______��
���𰸡�
��1�� �в������ƣ�
��2��b
��3�� ����ƿ�м���������Ũ���ᣬ��ҡ���»�������һ������Ũ���ᣬ��ȴ��
��4��ѹǿ��С������ �������ڱ��нۺ�ɫ�������� ����������ȥ�ƾ���
��5�� abc
��6��11.9%
��������
�����������1��ʵ�����а��ʱ�������ɷ�ĩ��Ҫ�IJ����������в������ƣ�����2���ǻ������п����¶�����ʵķ�ΧΪ60����65����ѡb����3��������������ȡ�����ᡱ�ľ�������ǣ�����ƿ�м���������Ũ���ᣬ��ҡ���»�������һ������Ũ���ᣬ��ȴ�����ɽ�Ũ����ӵ�Ũ�����У���4��ͼ2�У���ƿA�г�����������ѹ���ã����ܷ�ֹװ����ѹǿ���������¹ʣ����ܷ�ֹѹǿ��С����������2-����-1��3-�����ӵ��۵�Ϊ87.8��������ֱ��������C�е��������������ڱ��нۺ�ɫ������������Ӧ����ʱֹͣ����Ӧ�ô�����ͨ������Ȼ���ٳ�ȥ�ƾ��ƣ�������ƿA��ѹǿ��С����������5�� ���������Ƿ�����ᴿ�л�����ᴿ���ʱ���߱����ܻ�������ˮ�����������롢�ڷ�������ˮ��������ѧ��Ӧ������һ���Ļӷ��ԣ�abc��ȷ����6�������ṩ�ļ䱽���ӵ�����Ϊ71.5g�������ʵ���Ϊ71.5/110mol�������ϵõ�71.5/110mol2-����-1��3-�����ӣ����۲���Ϊ��71.5/110*155=100.75g������Ϊ��12.0/100.75=11.9%


 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���Ի�ͭ������Ϊԭ����ȡͭ������������¹��գ�ԭ�ϵ��ۺ������ʽϸߡ�����Ҫ������������ش��������⣺
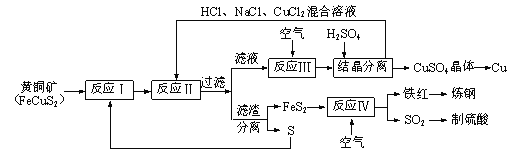
��1����Ӧ������ӷ���ʽΪ4CuCl2��+O2+4H+��4Cu2+��8Cl��+2H2O��CuCl2����ͭԪ�صĻ��ϼ�Ϊ______���÷�Ӧ�е���������___________��
��2��һ���¶��£��ڷ�Ӧ�����õ���Һ�м������ᣬ������������ͭ�������������Ȼ�ͭ��������ԭ��������____________________________________________________��
��3������ʱ���ɽ�����Ͷ�����ڵ������У��Խ��������ĺ�̼�����ù�������Ҫ��Ӧ�Ļ�ѧ����ʽ��______________________________________________________________��
��4��SO2β��ֱ���ŷŵ���������ɻ�����Ⱦ�ĺ����_____________________________��
��5����֪��Ӧ������ӷ���ʽΪCu2++CuS+4Cl����2CuCl2����S����Ӧ��IJ���Ϊ_____________��_____________�����ѧʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��


����ʵ��������
��1��ʵ����Ϻ���������ˮ������Ϊ___g��������ƿ������һ�����Σ�������Ϊ___g��
��2�����ɵ�ˮ����Ԫ�ص�����Ϊ____g��
��3�����ɵ�CO2��̼Ԫ�ص�����Ϊ_____g��
��4����̬��ʯȼ����̼Ԫ������Ԫ�ص�������Ϊ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л��ϳ��г��õ���/����̿����������ʹ�ã��ᱻ�����л��������������Ⱦ��ʧȥ���ԣ���Ϊ�ϴ�����һ���ɷϴ�����ȡPdCl2�Ĺ����������£�

��ش��������⣺
��1��������1����Ŀ����___________________________��
��2��д���״�(HCOOH)��PdO��Ӧ�Ļ�ѧ����ʽ______________________��
��3���ڡ���pHΪ8-9����Ϊ�˳�ȥ��Ԫ�أ�д�����������ӷ���ʽΪ______________________��
��4������Ũ������ϴ��Ŀ����___________________��
��5��д��֤��������2���й����ѷֽ���ȫ��ʵ�����___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±��г���A��R 9��Ԫ�������ڱ��е�λ�ã�

��1��FԪ�ص�����Ϊ ������B��ԭ�ӽṹʾ��ͼ�� ��
��2����9��Ԫ���У���ѧ��������õ���_______���Ⱦ��зǽ���Ԫ�ص�ijЩ���ʣ��ֿ��ܾ��н���Ԫ�ص�ijЩ���ʵ�Ԫ���� ������Ԫ�ط��ţ�
��3��A��D��E����Ԫ�ص�����������γɵľ��������ڷ��Ӿ������ ��A��B��Ԫ�ص����������۵�ϸߵ��� ��C��D��F�γɵ��⻯������й��ۼ��ļ�����ǿ������˳��Ϊ �����ѧʽ��
��4����ҵ����D��һ�ֵ���Ϊԭ������E���ʴ�Ʒ�Ļ�ѧ����ʽΪ ��
��5��G��H��ԭ��������� ���������֣�
��6��д��һ����֤��G��H���õĻ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ŵ����(fenofibrate)�ǽ���ͬ��������������ҩ�����һ���ϳ�·�����£�

��֪�����������л�����Һ���������������£�����![]() ȡ����
ȡ����
![]()
��1��B������Ϊ_______________��
��2��C���������ŵ�����Ϊ___________��
��3��д���������ʵĽṹ��ʽb_____________��F__________________��
��4��д��G��H�ķ�Ӧ����ʽ_______________��
��5��д��ͬʱ��������������D��ͬ���칹��ṹͲʽ____________��
���ܷ���������Ӧ���ں�5�ֲ�ͬ����������������л��
1 mol���л����������NaOH�����ʵ���Ϊ_______________��
��6����2-����ϩΪԭ���Ʊ�E����ƺϳ�·��(�����Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ȩ������ͭ�������ڵ������£����Ա��������������ᡣ���ݴ�ԭ�����ʵ���Ƶò����Թ�C���ռ�������������Һ(��ͼ��ʾ���Թ�A��װ��40������ȩˮ��Һ������ͭ��ĩ���Թ�C��װ����������ˮ���ձ�B��װ��ijҺ��)����֪��60����80��ʱ��˫�����������������ɷ�����ȩ��������Ӧ����������ʮ���η�Ӧ������ȫ���й����ʵķе���±���


��ش��������⣺
��1���Թ�A����60����80��ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ(ע����Ӧ����)____________________________________��
��2����ͼ��ʾ��ʵ��IJ�ͬ�Σ���Ҫ�����¶ȼ����Թ�A�ڵ�λ�ã���ʵ�鿪ʼʱ�¶ȼ�ˮ�����λ��Ӧ���Թ�A �ķ�ӦҺ�У�Ŀ����____________________�����Թ�A�ڵ���Ҫ��Ӧ��ɺ��¶ȼ�ˮ�����λ��Ӧ���Ե����Թ�A ��֧�ܿڴ���Ŀ����____________________
��3���ձ�B��������ʹ����A�ڵķ�ӦҺ�������ȷ�����Ӧ���ձ�B��ʢװ��Һ�������____________(�������������)��
��4����������Թ�C���Ƿ��в������ᣬ���������ṩ��ҩƷ�н���ѡ�����һ������ʵ�鷽�������ṩ��ҩƷ�У�pH��ֽ����ɫ��ʯ����ֽ����ɫ�Ĵ���Ǧ��ֽ��̼�����Ʒ�ĩ��ʵ��������ѡ���÷���Ϊ_______________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ⱥ���ĺϳ�·�����£�

��֪��![]()
��1��B�Ľṹ��ʽΪ_____________��A��ϵͳ����Ϊ_____________��
��2���ںϳ������Ⱥ������漰�ķ�Ӧ�У����ڼӳɷ�Ӧ����___________(�����)��
��3��д����Ӧ���Ļ�ѧ����ʽ___________________��
��4��C��ͬ���칹���ж��֣�д��ͬʱ�����������ʵ�ͬ���칹��Ľṹ��ʽ___________________��
a������FeCl3��Һ������ɫ��Ӧ b���ܷ���������Ӧ c���˴Ź�������ͼ���������
��5��д����![]() �Ʊ��߷��ӻ�����
�Ʊ��߷��ӻ�����![]() �ĺϳ�·������ͼ(���Լ���ѡ)���ϳ�����ʾ��ͼ���£�
�ĺϳ�·������ͼ(���Լ���ѡ)���ϳ�����ʾ��ͼ���£�![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ˮ�к������(CN-)��������Ũ���������ڹ����ŷű��涨�ķ�Χ����Ҫ�������账�����û�ѧ��������Ʒ�ˮ�е��軯�һ����ü����軯����˫��ˮ��������
I�������Ȼ������ڷ�ˮ�м���NaOHʹNi2+����Ni(OH)2������ͬʱ��NaClO��CN-����Ϊ�����ʣ����˷���ClO-�ڼ��������»��Ni2+��Ӧ��Ni(OH)3������Cl-���������ģ���Ӧ�����ӷ�Ӧ����ʽΪ_______________________________________��
����˫��ˮ�������ļ������������£�

��1����Ϸ�Ӧ���У��ڼ���������H2O2����CN-ȴ�����Ni2+��Ӧ���˷�Ӧ�����ӷ���ʽΪ______________��H2O2������������Ϊ25��2�ı���������ˮ�е��л����Ӽ������ͷ�ˮ��CODֵ��
��2������������У����뽹��������(Na2S2O5)��ԭ��ȥ������H2O2�������Ӱ����������������������ԭ��________________________________��
��3�����ˮ�м���30%H2O2(�ܶ�Ϊ1.11g/mL)������Ϊ1mL/L������ǰ��CN-���л����Ӽ�������Ũ�����±���ʾ����������H2O2�������ֽ����Һ����ı仯����������ڳ�������������ټ��뽹���� ����(Na2S2O5)����Ϊ_________g/L(������������С�������λ)��д��������̡�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com