
ij��ѧ�С��Ϊ̽����������ȡ��������Ϊԭ�Ͻ����ض���Ӧ��������±ߵ�ʵ��װ��ʾ��ͼ��
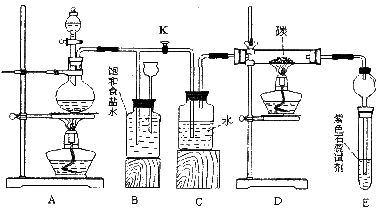
(1)Ϊ�����װ�õ������ԣ�������ͬѧ�ֱ��������������������
��ͬѧ����A�����ȣ�Ȼ����������Եļ������۲�B��C��E�е��й���������жϡ�
��ͬѧ���ȹر�K����ȼA���ľƾ��ƣ��۲�B�е��й���������жϣ��ٴ�K����ȼD���ľƾ��ƣ�����C��E�е��й���������жϡ�
�������������ͬѧ�������ĸ����ã�Ϊʲô?
(2)ʵ�鿪ʼʱ���ȵ�ȼA���ľƾ��ƣ�������K����C12��������װ�ã��ٵ�ȼD���ƾ��ƣ�������Eװ�á�C12ͨ��cƿ���ٽ���D��Dװ�õ�Ӳ�ʲ�������ʢ��̿�ۣ�����������ԭ��Ӧ�������ΪCO2��HCl��
��д��D�з�Ӧ�Ļ�ѧ����ʽ��__________��װ��C��������___________
(3)��E������ɫʯ����Һ����ɫ����ɫ���ɫ���ٱ�Ϊ��ɫ����ԭ����____________
(4)����E���Թ�����Һ��Ϊ����ʯ��ˮ����Ӧ����������Ϊ_______________
���������������Ҫԭ����________________(�û�ѧ����ʽ��ʾ)
(5)D����Ӧ��Ϻر�K����ȥ�ƾ��ƣ��������ȵ����ã�A������C12��������ʱB�е�������_________��B��������_____________
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A��B��C��D��E��5ֻС��ƿ�зֱ��������ϸ��˿������ʳ��ˮ��ϸ��˿��������ˮ��ϸ��˿����ʳ��ˮ��ȫ��û��ϸ��˿�Լ�����ˮ��ȫ��û��ϸ��˿��Ȼ��װ�����ͼ15-34��ʾ��5��װ�ã�ÿ��һ��ʱ�����������ˮ�������ĸ߶ȣ�������±���ʾ��������������Ϊ����ˮ�������ĸ߶ȣ���λ��cm):

ͼ15-34
ʱ��/h | 0 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
Aƿ��ʢ������˿�� | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Bƿ��ʢմ��ʳ��ˮ����˿�� | 0 | 0.4 | 1.2 | 3.4 | 5.6 | 7.6 | 9.8 |
Cƿ��ʢմ����ˮ����˿�� | 0 | 0 | 0 | 0.3 | 0.8 | 2.0 | 3.5 |
Dƿ��ʢ��ȫ��û��ʳ��ˮ�е���˿�� | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Eƿ��ʢ��ȫ��û����ˮ�е���˿�� | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
��1���������Ϊ��̽�������⣬��Ľ����ǣ�д�������ƣ�������������������������������?
��2��ʵ��ǰ����μ����ʵ��װ�õ������ԣ�����������������������������������������
��3��ͨ��ʵ��õ������ݣ����Եõ��Ľ���������������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)Ϊ�����װ�õ������ԣ�������ͬѧ�ֱ��������������������
��ͬѧ����A�����ȣ�Ȼ����������Եļ������۲�B��C��E�е��й���������жϡ�
��ͬѧ���ȹر�K����ȼA���ľƾ��ƣ��۲�B�е��й���������жϣ��ٴ�K����ȼD���ľƾ��ƣ�����C��E�е��й���������жϡ�
�����____________________________(��ס����ҡ�)ͬѧ���������á�
(2)ʵ�鿪ʼʱ���ȵ�ȼA���ľƾ��ƣ�������K����Cl2��������װ�ã��ٵ�ȼD���ƾ��ƣ�������Eװ�á�Cl2ͨ��Cƿ���ٽ���D��Dװ�õ�Ӳ�ʲ������ڷ�����Ӧ�������ΪCO2��HCl��
��д��D�з�Ӧ�Ļ�ѧ����ʽ��____________________��װ��C��������____________________________��
(3)��E������ɫʯ����Һ����ɫ�ȱ�Ϊ��ɫ���ٱ�Ϊ��ɫ����ԭ����______________��
(4)����E���Թ�����Һ��Ϊ����ʯ��ˮ����Ӧ������______________(��С����ޡ�)��ɫ���������������������Ҫԭ����(�û�ѧ����ʽ��ʾ)______________��
(5)D����Ӧ��Ϻر�K����ȥ�ƾ��ƣ��������ȵ����ã�A������Cl2��������ʱB�е�������______________��B��������____________________________________��
(6)��ʵ��װ�õ�һ������ȱ����__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
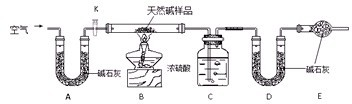
��10�֣�ij����С��Ϊ̽��CO2�������NaOH��Һȷʵ�����˻�ѧ��Ӧ���ס��ҡ�����λͬѧ�������������ʵ��װ�ã���ش��������⣺
(1)��װ����CO2�������NaOH��Ӧ�Ļ�ѧ����ʽ ��
(2)��װ��ʵ�������� �����Ͳ�����ʵ�������ԭ��
��
(3)�ס��ҡ���ͬѧ��Ƶ����������У���һ��������ʵ�ʲ����а�ȫ�Դ������⣬�÷����� (��ס��һ��)��ԭ����
��
(4)�����һ��ʵ��������ɵIJ���Na2CO3�е������ӡ�(�����������衢�����Լ���ʵ������ͽ��ۡ�)
(5)ʵ������������44.8 L(��״̬)CO2 �����ú�CaCO3 90%ʯ��ʯ�����������ᷴӦ��������Ҫ����ʯ��ʯ g(�������һλС��)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com