
����10�֣���֪�ϳɰ���ӦΪ��N2
+ 3H2 2NH3����һ���¶��£���2L�ܱ������У�����2molN2��5molH2��һ��������ʹ֮��Ӧ������2min��ﵽƽ��״̬�����NH3Ϊ0.4mol����
2NH3����һ���¶��£���2L�ܱ������У�����2molN2��5molH2��һ��������ʹ֮��Ӧ������2min��ﵽƽ��״̬�����NH3Ϊ0.4mol����
��1����N2��ʾ��2min�ڸ÷�Ӧ�ķ�Ӧ���ʣ�
��2����ʱ������ת���ʣ�
��3��ƽ��ʱNH3�����������������1λС����
��4��ƽ��ʱ�����е�ѹǿ�뿪ʼѹǿ֮�ȣ�
��5��ƽ��ʱ�����������ƽ��Ħ��������
��1��0.05 mol/L.min ��2��12% ��3��6.7% ��4��33/35 ��5��10g/mol
��������N2
+ 3H2 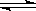 2NH3
2NH3
2mol 5mol 0
0.2mol 0.6mol 0.4mol
1.8mol 4.4mol 0.4mol
V(N2)=0.2mol��2L.2min=0.05 mol/L.min �}H2=0.6mol��5mol=12%
NH3�G=0.4mol��6.6mol=6.7% p2��p1=n2��n1=6.6mol��7mol=33/35
M=m��n=(28��2+5��2)��6.6mol=10g/mol



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����10�֣���֪��һ�������¿�ʵ������ת�䣺
���Ʒ��dz��õ��л��ϳ���·��Ʒ�������ν���Ʒ����ǴӲ������Ƴ�ԭ�ϣ���Ƴ������ĺϳ���·�������ƹ����У���Ҫ����Ѱ����˳���ϳ�Ŀ����ӵ��м��л��ֱ��ѡ�����ʵ���ʼԭ�ϡ��������Dz������Ʒ��������ˮ����ĺϳ���·��
��д���ʵ����м��л���A��B��C��D��E�Ľṹ��ʽ��
A B
C D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09��10�������������и߶���ѧ��ĩ���Ի�ѧ�� ���ͣ������
����10�֣���֪��һ�������¿�ʵ������ת�䣺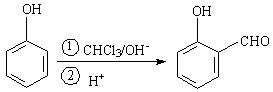
���Ʒ��dz��õ��л��ϳ���·��Ʒ�������ν���Ʒ����ǴӲ������Ƴ�ԭ�ϣ���Ƴ������ĺϳ���·�������ƹ����У���Ҫ����Ѱ����˳���ϳ�Ŀ����ӵ��м��л��ֱ��ѡ�����ʵ���ʼԭ�ϡ��������Dz������Ʒ��������ˮ����ĺϳ���·��
��д���ʵ����м��л���A��B��C��D��E�Ľṹ��ʽ��
A B
C D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ϻ��г����ظ����߿�ģ�⿼�ԣ���ģ����ѧ�Ծ��������棩 ���ͣ������
����10�֣���������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����á���ҵ�Ͽ����ý�̿��ˮ�����ڸ����µķ�Ӧ���ˮú�����ϳɶ����ѡ���ش��������⣺
��1������ˮú������Ҫ��ѧ��Ӧ����ʽΪ������������������������������������������
��2����ú�����������в������к�����H2S��Na2CO3��Һ���գ�����������ʽ�Σ��÷�Ӧ�Ļ�ѧ����ʽΪ��������������������������������������������������������
��3��������ˮú���ϳɶ����ѵ�������Ӧ���£�
������ 2H2(g) CO(g)
CO(g) CH3OH(g)
CH3OH(g) 90.8 kJ
90.8 kJ
������ 2CH3OH(g) CH3OCH3(g)
CH3OCH3(g) H2O(g)
H2O(g) 23.5 kJ
23.5 kJ
������ CO(g) H2O(g)
H2O(g) CO2(g)
CO2(g) H2(g)
H2(g) 41.3 kJ
41.3 kJ
�����ܷ�Ӧ��3H2(g) 3CO(g)
3CO(g) CH3OCH3(g)
CH3OCH3(g) CO2(g)
CO2(g) Q������Q
Q������Q ����������kJ��
����������kJ��
����һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��
����������������ĸ���ţ���
a�����¸�ѹ b��������� c������CO2��Ũ�� d������CO��Ũ��
��4������֪ij�¶��·�Ӧ��2CH3OH(g) CH3OCH3(g)
CH3OCH3(g) H2O(g)��һ�ܱ������н��е�10minʱǡ�ô�ƽ�⣬��ø���ֵ�Ũ�����£�
H2O(g)��һ�ܱ������н��е�10minʱǡ�ô�ƽ�⣬��ø���ֵ�Ũ�����£�
|
���� |
CH3OH |
CH3OCH3 |
H2O |
|
Ũ�ȣ�(mol��L |
0.44 |
0.6 |
0.6 |
�����Ƚϴ�ʱ�����淴Ӧ������ֵ����λ��ͬ���Ĵ�С�� (�״�) ������
(�״�) ������ (ˮ)�������������������)��
(ˮ)�������������������)��
������Ӧ��ʼʱ (CH3OH)
(CH3OH) ������������
������������
������ʱ���ڵ�ƽ����Ӧ���� (CH3OH)
(CH3OH) ������������
������������
�������¶��·�Ӧ��ƽ�ⳣ��ֵΪ����������������ȷ��0.01��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10�������������и߶���ѧ��ĩ���Ի�ѧ�� ���ͣ������
����10�֣���֪��һ�������¿�ʵ������ת�䣺
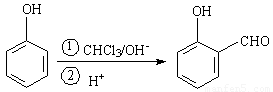
���Ʒ��dz��õ��л��ϳ���·��Ʒ�������ν���Ʒ����ǴӲ������Ƴ�ԭ�ϣ���Ƴ������ĺϳ���·�������ƹ����У���Ҫ����Ѱ����˳���ϳ�Ŀ����ӵ��м��л��ֱ��ѡ�����ʵ���ʼԭ�ϡ��������Dz������Ʒ��������ˮ����ĺϳ���·��

��д���ʵ����м��л���A��B��C��D��E�Ľṹ��ʽ��
A B
C D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����10�֣���֪��һ�������¿�ʵ������ת�䣺
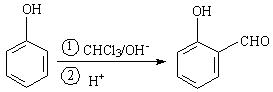
���Ʒ��dz��õ��л��ϳ���·��Ʒ�������ν���Ʒ����ǴӲ������Ƴ�ԭ�ϣ���Ƴ������ĺϳ���·�������ƹ����У���Ҫ����Ѱ����˳���ϳ�Ŀ����ӵ��м��л��ֱ��ѡ�����ʵ���ʼԭ�ϡ��������Dz������Ʒ��������ˮ����ĺϳ���·��
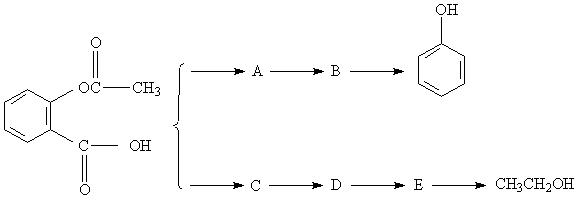
��д���ʵ����м��л���A��B��C��D��E�Ľṹ��ʽ��
A B
C D
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com