
���� ��1�������Ȼ�ѧ����ʽ��˹���ɼ������軯ѧ����ʽ��
��2���绯��Ӧ��Ԫ������Ϊ�������Σ���Ԫ�ػ��ϼ�Ϊ+1�ۣ����͵�ֻ��Ϊ���ۣ���Ϊ�⻯�
��3��H3PO4?H++H2PO4-K1=7.1��10-3��H2PO4-?H++HPO42-K2=6.3��10-8��HPO42-?H++HPO43-K3=4.2��10-13����һ�����������H+����һ���������������ã�Ϊ��þ����ܴ���NaH2PO4����H2PO4-Ũ�������K1��K2���Լ�pH=-lgc��H+�����㣻
����Na2HPO4��Һ�м���������CaCl2��Һ��HPO42-���Ӻ�Ca2+���ӷ�Ӧ���ɳ������ٽ�HPO42-�ĵ��룬��Һ��ʾ���ԣ�
��4��CuԪ�صĻ��ϼ���+2�۽��͵�+1�ۣ�CuSO4����������P4������Ԫ����0�۽��͵�-3�ۣ�������Ԫ����0�����ߵ�+5�ۣ���Ԫ�صĻ��ϼۼ������ֽ��ͣ�����P4�������������ǻ�ԭ��������11molP4�μӷ�Ӧ������5mol��P4����������60mol����ͭ����������ֻ��6mol��P4����ԭ������ϵ����غ������
��� �⣺��1����4Ca3��PO4��2��s��+10C��s��=12CaO��s��+2P4��s��+10CO2��g����H1=+Q1kJ•mol-1
��CaO��s��+SiO2��s��=CaSiO3��s����H2=-Q2 kJ•mol-1
��CO2��g��+C��s��=2CO��g����H3=+Q3kJ•mol-1
�����Ȼ�ѧ����ʽ��˹���ɼ���õ��١�$\frac{1}{2}$+��+�ڡ�6�õ���ѧ����ʽΪ��2Ca3��PO4��2+6SiO2+10C$\frac{\underline{\;����\;}}{\;}$6CaSiO3+P4+10CO��
�ʴ�Ϊ��2Ca3��PO4��2+6SiO2+10C$\frac{\underline{\;����\;}}{\;}$6CaSiO3+P4+10CO��
��2���������ȵ�Ũ����������Һ��᪻��õ�һ�ִ������Σ�KH2PO2����һ�����壬�绯��Ӧ��Ԫ������Ϊ�������Σ���Ԫ�ػ��ϼ�Ϊ+1�ۣ����͵�ֻ��Ϊ���ۣ���Ϊ�⻯��PH3��
�ʴ�Ϊ��PH3��
��3��Ϊ��þ����ܴ���NaH2PO4�����������ᡢ��������ƵĻ�����Һ������Һȫ��Ϊ������Һʱ�������Ե�һ������Ϊ��������H3PO4?H++H2PO4-K1=7.1��10-3��PH=-lgc��H+��=3-lg7.1��2.1������Һȫ��ΪNaH2PO4��Һʱ��H2PO4-?H++HPO42-K2=6.3��10-8������pH=-lgc��H+��=8-lg6.3��7.2��
����pHӦ���ƽ���2.1��7.2֮�䣬HPO42-���Ӽ��ܷ����������ܷ���ˮ�⣬���뷴ӦʽΪHPO42-?PO43-+H+��ˮ�ⷴӦʽΪHPO42-+H2O?H2PO4-+OH-����Һ�ʼ��ԣ�˵��ˮ��̶ȴ��ڵ���̶ȣ�����HPO42-���Ӻ�Ca2+���ӷ�Ӧ���ɳ�����3Ca2++2HPO42-�TCa3��PO4��2��+2H+���ٽ�HPO42-�ĵ��룬��Һ�������ԣ�
�ʴ�Ϊ��2.1��7.2��3Ca2++2HPO4-=Ca3��PO4 ��2��+2H+��
��4��CuԪ�صĻ��ϼ���+2�۽��͵�+1�ۣ�CuSO4����������P4������Ԫ����0�۽��͵�-3�ۣ�������Ԫ����0�����ߵ�+5�ۣ���Ԫ�صĻ��ϼۼ������ֽ��ͣ�����P4�������������ǻ�ԭ��������11molP4�μӷ�Ӧ������5mol��P4����������60mol����ͭ����������ֻ��6mol��P4����ԭ����
���ɵ����غ��֪����60mol��CuSO4�μӷ�Ӧ��60molCuSO4�õ�60mol���ӣ�1molP4�μӷ�Ӧʧȥ20mol���ӣ�����60molCuSO4�������������ʵ�����$\frac{60}{20}$=3mol��
�ʴ�Ϊ��3mol��
���� ���⿼������ϵĶ����жϺͼ��㡢��˹���ɵ�Ӧ�á�������ԭ��Ӧ����ת�Ƽ��㡢���ӷ���ʽ����д�ȣ���Ŀ�漰��֪ʶ��϶࣬������ѧ���ķ��������ͼ��������Ŀ��飬��Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
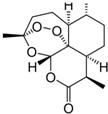 �ҹ�Ůҩѧ����������Ϊ��ű��ҩ�����صĵ�һ�������ٻ�2015��ŵ��������ѧ��ҽѧ���������صĽṹ��ͼ��ʾ�����й��������ص�˵��������ǣ�������
�ҹ�Ůҩѧ����������Ϊ��ű��ҩ�����صĵ�һ�������ٻ�2015��ŵ��������ѧ��ҽѧ���������صĽṹ��ͼ��ʾ�����й��������ص�˵��������ǣ�������| A�� | �����صĻ�ѧʽΪC15H22O5 | |
| B�� | �����ص�ͬ���칹������з����廯���� | |
| C�� | �����ؿ���NaOH��Һ����ˮ�ⷴӦ | |
| D�� | �����ؿ�������ˮ��ֲ����ȡ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ȼ�ŵľƾ��ƣ�������ʪĨ������ | |
| B�� | ʵ������ȡ���������β������װ�ã��ɲ�����ͨ����н��� | |
| C�� | ��ʢ��Һ����Թܼ���ʱ��Ҫ�����ƶ��Թܻ�������Ƭ | |
| D�� | Ũ��Һ����Ƥ���ϣ������ô���ˮ��ϴ��Ȼ��Ϳ��������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���շϾɵ�� | |
| B�� | ��ǿ����ȼ���̻����� | |
| C�� | ֹͣʹ�ú�Ǧ���� | |
| D�� | �������̴ѼӸߣ�������Χ������Ⱦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
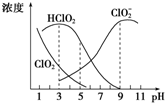 ����������һ�ָ�Ч��������Ư������Ҫ�����ġ�ֽ����Ư�ף��������ƣ�NaClO2������Һ�п�����ClO2��HClO2��ClO${\;}_{2}^{-}$��Cl-�ȣ�����HClO2��ClO2������Ư�����ã���ClO2���ж����壮���ⶨ��25��ʱ����ֺ�����pH�仯�����ͼ��ʾ��Cl-û�л������������з���������ǣ�������
����������һ�ָ�Ч��������Ư������Ҫ�����ġ�ֽ����Ư�ף��������ƣ�NaClO2������Һ�п�����ClO2��HClO2��ClO${\;}_{2}^{-}$��Cl-�ȣ�����HClO2��ClO2������Ư�����ã���ClO2���ж����壮���ⶨ��25��ʱ����ֺ�����pH�仯�����ͼ��ʾ��Cl-û�л������������з���������ǣ�������| A�� | ���������ڼ��������½��ȶ� | |
| B�� | 25��ʱ��HClO2�ĵ���ƽ�ⳣ������ԼΪKa=10-6 | |
| C�� | 25��ʱ��ͬŨ�ȵ�HClO2��Һ��NaClO2��Һ�������ϣ�����Cl-����������Һ����c��HClO2��+2c��H+���Tc��ClO${\;}_{2}^{-}$��+2c��OH-�� | |
| D�� | ʹ�ø�Ư�������pHΪ3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
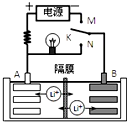 ��ͼ��һ�ֿɳ�������ӵ�س䡢�ŵ�Ĺ���ʾ��ͼ���õ�صķ�ӦʽΪ��
��ͼ��һ�ֿɳ�������ӵ�س䡢�ŵ�Ĺ���ʾ��ͼ���õ�صķ�ӦʽΪ��| A�� | K��N���ʱ��A��Ϊ�������õ缫��ӦʽΪ��LixC6-xe��=C6+xLi+ | |
| B�� | �õ�صı��������õ�������缫��﮵�صı�������ͬ | |
| C�� | K��M���ʱ��A������������Ӧ��LiMnO2-xe��=Li1-xMnO2+xLi+ | |
| D�� | K��N���ʱ��Li+��A����Ǩ�Ƶ�B���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
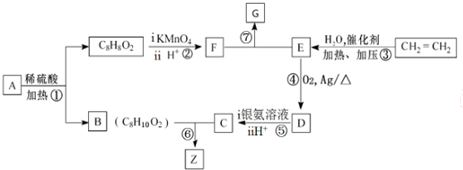
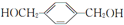 ������������������Ϊ�ǻ���
������������������Ϊ�ǻ��� ��
��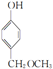 ��
��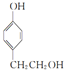 ��
��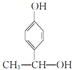 ��
��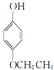 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
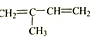 ��
��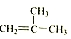
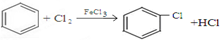 ��
�� ��
�� D��
D��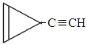 e��
e��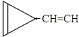 ��
�� ��
��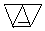 ��ֻ����һ���Ľṹ��ʽ����
��ֻ����һ���Ľṹ��ʽ�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com