
�����£����и��������б�ֵΪ2:1����(����)
A��K2SO3��Һ��c(K��)��c(SO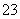 )֮��
)֮��
B��0.2mol·L��1��CH3COOH��Һ��0.1mol·L��1��������c(H��)֮��
C��pH��7�İ�ˮ��(NH4)2SO4�Ļ����Һ�У�c(NH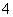 )��c(SO
)��c(SO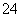 )֮��
)֮��
D��pH��12��Ba(OH)2��Һ��pH��12��KOH��Һ�����ʵ����ʵ���Ũ��֮��
�𰸡�C
������K2SO3��Һ�У�����SO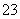 ˮ������HSO
ˮ������HSO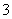 ������K2SO3��Һ��c(Na��):c(SO
������K2SO3��Һ��c(Na��):c(SO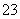 )֮�ȴ���2:1������CH3COOH�����ᣬ����CH3COOHCH3COO����H����������ǿ�ᣬ����Һ��ȫ�����룬������Һ��c(H��)֮��С��2:1��pH��7�İ�ˮ��(NH4)2SO4�Ļ����Һ�У����ݵ���غ��У�c(NH
)֮�ȴ���2:1������CH3COOH�����ᣬ����CH3COOHCH3COO����H����������ǿ�ᣬ����Һ��ȫ�����룬������Һ��c(H��)֮��С��2:1��pH��7�İ�ˮ��(NH4)2SO4�Ļ����Һ�У����ݵ���غ��У�c(NH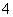 )��c(H��)��2c(SO
)��c(H��)��2c(SO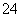 )��c(OH��)����c(H��)��c(OH��)������c(NH
)��c(OH��)����c(H��)��c(OH��)������c(NH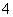 )��2c(SO
)��2c(SO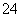 )����c(NH
)����c(NH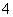 ):c(SO
):c(SO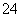 )��2:1��pH��12��Ba(OH)2��Һ�У�����Ba(OH)2�����ʵ���Ũ��Ϊ
)��2:1��pH��12��Ba(OH)2��Һ�У�����Ba(OH)2�����ʵ���Ũ��Ϊ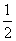 ��0.01mol·L��1��pH��12��KOH��Һ������KOH�����ʵ���Ũ��Ϊ0.01mol·L��1��Ba(OH)2��KOH��Һ�����ʵ����ʵ���Ũ��֮��Ϊ1:2��
��0.01mol·L��1��pH��12��KOH��Һ������KOH�����ʵ���Ũ��Ϊ0.01mol·L��1��Ba(OH)2��KOH��Һ�����ʵ����ʵ���Ũ��֮��Ϊ1:2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������֬����;����(����)
�������Ӫ�����ʡ�����ȡ����������ȡ���͡�����ȡ�� ��֬���ᡡ����ȡ����
��֬���ᡡ����ȡ����
A���٢ڢ� B���٢ۢ� C���ڢۢܢ� D���٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ʺ�����һ����Դ�������ӵ��ڼ�������������ʳ���е�ɫ����ת���õ���
����˵������ȷ���� (����)
A��ɫ��������д��ڰ������Ȼ������γ����Σ����нϸߵ��۵�
B����ɫ����ˮ��Һ�У���ͨ��������Һ��pHʹ���γɾ�������
C����һ�������£�ɫ����ɷ������۷�Ӧ
D���ʺ�����ɫ����ṹ���ƣ�Ҳ�������Ի����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
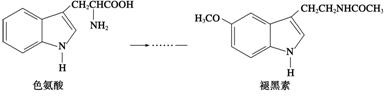
ij��ѧ����С��ͨ��ʵ���о�NO2�����ʣ�
��֪��2NO2+2NaOH═NaNO3+NaNO2+H2O
����ͼ1��ʾװ��̽��NO2�ܷ�NH3��ԭ��K1��K2Ϊֹˮ�У��г̶ֹ�װ����ȥ��
��1��Eװ������ȡNO2��Ӧ�����ӷ���ʽ��
��2����ʵ������ȡ����ʱ��ֻ��һ���Լ���������������ѡ ȡ �� ��
ȡ �� ��
a��NH4HCO3 b��NH4Cl c��Ũ��ˮ
��3����NO2�ܹ���NH3��ԭ��Ԥ�ڹ۲쵽Cװ���е�������
��4����ʵ��װ�ô���һ�����Ե�ȱ����
��5��̽��NO2�ܷ���Na2O2����������ԭ��Ӧ��Ϊ����֤NO2�ܱ�Na2O2��������С��ͬѧѡ��B��D��Eװ�ã���B�е�ҩƷ����ΪNa2O2����ѡFװ�ã���ͼ2��ʾ����������װ������ʵ�飮װ�õĺ�������˳����
��6��ʵ������У�Bװ���е���ɫ��ĩ�� ����ɰ�ɫ�������飬�ð�ɫ����Ϊ��������������������ɣ��Ʋ�Bװ���з�Ӧ�Ļ�ѧ����ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�����������ݣ���7.2��10��4����4.6��10��4����4.9��10��10���ֱ���������ĵ���ƽ�ⳣ��������֪��Щ��ɷ������·�Ӧ��NaCN��HNO2===HCN��NaNO2��NaCN��HF===HCN��NaF��NaNO2��HF===HNO2��NaF���ɴ˿��ж����������У���ȷ����(����)
A��HF�ĵ���ƽ�ⳣ���Ǣ�
B��HNO2�ĵ���ƽ�ⳣ���Ǣ�
C��HCN�ĵ���ƽ�ⳣ���Ǣ�
D��HNO2�ĵ���ƽ�ⳣ���Ǣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1.0mol·L��1NaOH��Һ�к�ijŨ��������Һʱ����pH������NaOH��Һ�������ϵ��ͼ��ʾ��ԭ������Һ�����ʵ���Ũ�Ⱥ���ȫ��Ӧ����Һ���������(����)
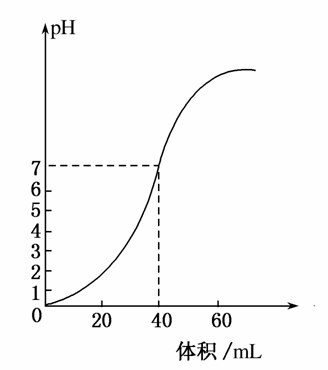
A��1mol·L��1,60mL
B��0.5mol·L��1,80mL
C��0.5mol·L��1,40mL
D��1mol·L��1,80mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���ijЩ����ȵ������˵���У�����ȷ����(����)
A�����ú�Ǧ������Ϊ��������͵�ȼ��Ч��
B���״���������ľƾ��Բ�������
C����ȩ��ijЩ����װ�β����ͷŵij���������Ⱦ��֮һ
D���������Դ�������������ƻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����С����Ƶ�ʵ������ȡ����������װ������ͼ��ʾ��A��ʢ��ŨH2SO4��B��ʢ���Ҵ�����ˮ�����ƣ�D��ʢ�б���̼������Һ��
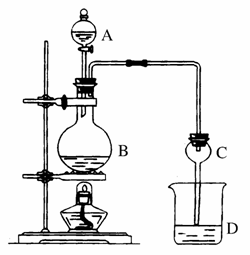
��֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2·6C2H5OH��
���й��л���ķе㣺
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е�(��) | 34.7 | 78.5 | 118 | 77.1 |
��ش�
(1)Ũ���������__________������ͬλ��18Oʾ�ٷ�ȷ����Ӧ����ˮ��������ԭ�ӵ��ṩ��(��18O��CH3CH OH��)��д���ܱ�ʾ18Oλ�õĻ�ѧ����ʽ_____________________________________ ___________________________________��
OH��)��д���ܱ�ʾ18Oλ�õĻ�ѧ����ʽ_____________________________________ ___________________________________��
(2)���θ����C��������______________������Ӧǰ��D�м��뼸�η�̪����Һ�ʺ�ɫ�������������ԭ����(�����ӷ���ʽ��ʾ)��________________________����Ӧ������D�е�������______________________��
(3)��D�з���������������г�����һ�������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ������______________���ټ�����ˮ�����ƣ�Ȼ����������ռ���Ʒ��������ʱ���¶�Ӧ������__________���ҡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��CaSO4����O2��ȼ��CO��Ӧ���ȿ����ȼ��Ч�ʣ����ܵõ��ߴ�CO2����һ�ָ�Ч����ࡢ���õ�����ȼ�ռ�������Ӧ��Ϊ����Ӧ����Ӧ�ں͢�Ϊ����Ӧ��
��1/4CaSO4(s)��CO(g)1/4CaS(s)��CO2(g)
��H1����47.3 kJ��mol��1
��CaSO 4(s)��CO(g)CaO(s)��CO2(g)��SO2(g)
4(s)��CO(g)CaO(s)��CO2(g)��SO2(g)
��H2����210.5 kJ��mol��1
��CO(g)1/2C(s)��1/2CO2(g)
��H3����86.2 kJ��mol��1
(1)��Ӧ2CaSO4(s)��7CO(g)CaS(s)��CaO(s)��6CO2(g)��C(s)��SO2(g)�Ħ�H��________(�æ�H1����H2�ͦ�H3��ʾ)��
(2)��Ӧ�١��۵�ƽ�ⳣ���Ķ���lgK�淴Ӧ�¶�T�ı仯������ͼ��ʾ����ϸ���Ӧ�Ħ�H������lgK��T���߱仯���ɣ�
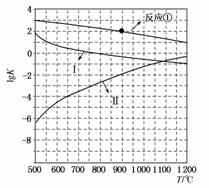
a)__________________________________________________________��
b)_________________________________________________________��
(3)��ʢ��CaSO4����պ����ܱ������г���CO����Ӧ����900 ��ﵽƽ�⣬cƽ��(CO)��8.0��10��5 mol��L��1������CO��ת����________(���Ը���Ӧ�����������λ��Ч����)��
(4)Ϊ���ٸ������ø�������CO2�����ڳ�ʼȼ������������________________________________________________________________________��
(5)�Է�Ӧ�������ɵ�CaSΪԭ�ϣ���һ�������¾�ԭ��������100%�ĸ��·�Ӧ��������CaSO4���÷�Ӧ�Ļ�ѧ����ʽΪ______________________����һ�������£�CO2����Զ��ױ���Ӧ�����䱽��������һ���Ȼ�������Ľṹ��ʽΪ________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com