
�ܺ�����άϵ���չ����Ҫǰ��֮һ��Ŀǰ����ʹ�õ���Ҫ�ܻ��ǻ�ʯȼ�ϡ���ش��������⣺
(1)��ʯȼ�ϰ���ú��ʯ�͡� (д������һ�ֻ�ʯȼ�ϵ�����)��ú̿���ɹ����У�����ȡ�Ĵ�ʩ��������������˹��ը�¹ʣ����������Ʋ���ʧ���û�ѧ����ʽ������ú����ƵƵ������˹��ը��ԭ��
��
(2)ú���������Ի���ʯ��Σ������Ŀǰú��������Ҫ��ú�е�̼��ˮ�����ķ�Ӧ��C��H2O(g)===CO��H2���÷�Ӧ��һ�����ȷ�Ӧ����Ӧ���������һ�����ɼ�Ъ���е�̼��ȼ��(�����ÿ�������)�ṩ�ģ�C��O2===CO2��
��������Ϊ������һ����Ӧ��Ҫ���ȣ�����ú�������������Ƕȿ����ò���ʧ������������ֹ۵�Ĵ������ڣ�
��
��ú����������Ϊ��ҵ�ϳɰ��ṩԭ������������ �������� �������Բ��� ���յõ������еĵ�����
(3)�ҹ�ú̿�����ʯ�ͺ���Ȼ���ʷḻ��ú������һ�����ʱ��ϡ�úҺ������Ҫ�����Ǽ��Һ���������Ƚ�ú����ΪH2��CO��CH4��Ȼ��ͨ����������̬����ת��ΪҺ̬��ͨ����ӷ��Ʊ���ȼ���к��б��������Ͳ�����ϩ������������д�ú���ϳ�ϩ���������Ļ�ѧ����ʽ��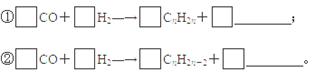
(1)��Ȼ����CH4��2O2�D��CO2��2H2O
(2)��ú������õ�����̬ȼ��ȼ��ʱ���������ʸߣ���Դ��ࡡ��̼��ˮ�����ķ�Ӧ��̼�ڿ�����ȼ�պ�ʣ������塡����Һ����
(3)��n��2n��1��nH2O����n��2n��1��1��nH2O
���������������1����ʯȼ�ϰ���ú��ʯ�͡���Ȼ������˹����Ҫ�ɷ��Ǽ��飬����ȼ�յĻ�ѧ����ʽΪCH4��2O2 CO2��2H2O��
CO2��2H2O��
��2������Ȼú�����������ȷ�Ӧ����ú������õ�����̬ȼ��ȼ��ʱ���������ʸߣ���Դ��ࣻ
�ںϳɰ���ҵ��ԭ�����ǵ���������������������̼��ˮ�����ķ�Ӧ������ˮú������������Ҫ�ɷ��ǵ�����������̼�ڿ�����ȼ�պ�ʣ���������ǵ����������ںϳɰ���ҵ��
��3���ٸ��������غ㶨�ɣ�CO��H2��Ӧ����ϩ���Ӧ��ˮ���ɣ�ϩ���ķ���ʽΪCnH2n������CO��ϵ����n����O�ĸ�����n����H2O��ϵ����n����H2��ϵ����2n��
��ͬ������ƽCO��H2���������Ļ�ѧ����ʽ��ϵ����������ͨʽCnH2n+2���ɵ�CO��ϵ����n��H2O��ϵ����n����H2��ϵ����2n+1��
���㣺���黯ʯȼ�ϵ��жϣ���ѧ����ʽ����д����ƽ��ú������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������л���Ľṹ�������йص�������ȷ����
| A��������ϩ����֬����ʹ����KMnO4��Һ��ɫ |
| B��ʯ�͵���Ҫ�ɷ�������ú����������Ƶý�̿��ú���͵Ȳ�Ʒ |
| C�����ۡ���ά�ص���ɶ������ã�C6H10O5��n��ʾ������Ϊͬ���칹�� |
| D��������Ҵ�������������Ӧ���ֶ��������Ʒ����û���Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���з�Ӧ���õ��л�����ֻ��һ�ֵ���( )
| A��������ļ����������ڹ��������µ�ȡ����Ӧ |
| B����ϩ���Ȼ���ļӳɷ�Ӧ |
| C��CH3��CH��OH����CH3��Ũ���������µ���ȥ��Ӧ |
| D���ױ���Һ�����廯�������������µ�ȡ����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��4�֣����У��ٶ�����̼ �ڼ��� �۶������� ��������ͭ��������ѧ֪ʶ����������������������ѡ����ʵ������������пո��У����ţ���
��1����Ȼ������Ҫ�ɷ��� ��
��2��ҽԺ��������Ҫ�õ��������� ��
��3���������ЧӦ��ȫ�������ů����Ҫ������ ��
��4��й©�������ж������������Ҫ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�л���Ľṹ��ʽΪ��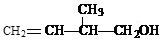
��1�����л��������������ŵ�������_______________________________________��
��2�����л�����Ӿ۷�Ӧ�����ò���Ľṹ��ʽΪ_______________________��
��3��д�����л�����������Ļ�ѧ����ʽ��ע����Ӧ������_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ϳ��л���G��C9H10O2���Ĺ������£��Իش������й����⡣
��1��A�ķ���ʽΪ ��
��2��C�еĹ����ŵ������� ��Cת��ΪD�ķ�Ӧ���� ��
��3��Eת��ΪF�Ĺ������ữ��Ŀ���� ��
��4��д��G������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��5����������������G��ͬ���칹����ĿΪ �֣�
�ٱ�������3��ȡ��������������ȡ������ͬ��
���ܹ������Ƶ�������Һ��Ӧ����������������������ԭ�ӹ������ֲ�ͬ�������������ʵĽṹ��ʽΪ �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�о�С���Լױ�Ϊ��Ҫԭ�ϣ���������·�ߺϳ�ҽҩ�м���F��Y��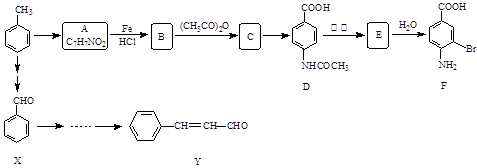
��֪��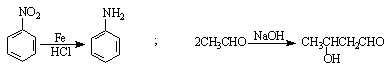
��ش��������⣺
��1�������й�F��˵����ȷ���� ��
| A������ʽ��C7H7NO2Br | B�����γ����� |
| C���ܷ���ȡ����Ӧ�����۷�Ӧ | D��1 mol�� F�����Ժ�2 mol NaOH��Ӧ |
 ��
��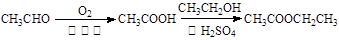
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�л���M�Ľṹ��ʽ��ͼ��ʾ��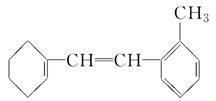
��1���л���M�ı����ϵ�һ�ȴ�����________�֡�
��2��1 mol M��������ˮ��ϣ�����Br2�����ʵ���Ϊ________mol��
��3��1 mol M������H2�ӳɣ�����H2________ mol��
��4�������й�M��˵���в���ȷ����________��
| A���ڴ����������£�M����Һ�巢��ȡ����Ӧ |
| B��Mʹ��ˮ��ɫ��ԭ������ϩʹ��ˮ��ɫ��ԭ����ͬ |
| C��M��ʹ����KMnO4��Һ��ɫ |
| D��M�ͼױ���Ϊͬϵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����3���������A�� CH2=CH-CH3 B�� C��CH3CH2OH
C��CH3CH2OH
��1��д��������A��C�еĹ����ŵ����� �� ��
��2��A�ڴ������������¾ۺ����ɾۺ���ķ�Ӧ����ʽΪ ����Ӧ����Ϊ ��
��3��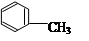 ��Ũ���������£���Ũ���Ṳ����100�淴Ӧ�Ļ�ѧ����ʽΪ�� ��
��Ũ���������£���Ũ���Ṳ����100�淴Ӧ�Ļ�ѧ����ʽΪ�� ��
��4��B���Ա����Ը�����������ɱ����ᣬд����������C������Ũ���Ṳ�ȷ���������Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com