
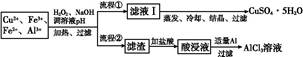
| Ąė×Ó | Fe3+ | Fe2+ | Al3+ | Cu2+ |
| pH·¶Ī§ | 2.2”«3.2 | 5.5”«9.0 | 4.1”«5.0 | 5.3”«6.6 |
 2Fe3++2H2O
2Fe3++2H2O
 2Fe3++2H2Oӣ
2Fe3++2H2O”£ Al(OH)3+3HCl,¼ÓČČÕō·¢Ź±,HCl»į»Ó·¢Ōģ³ÉĘ½ŗāÓŅŅĘ,×īÖÕµĆ²»µ½AlCl3”£
Al(OH)3+3HCl,¼ÓČČÕō·¢Ź±,HCl»į»Ó·¢Ōģ³ÉĘ½ŗāÓŅŅĘ,×īÖÕµĆ²»µ½AlCl3”£ Al(OH)3”ż,
Al(OH)3”ż, Fe(OH)3”ż;
Fe(OH)3”ż; Al
Al +2H2O,
+2H2O,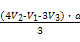 ,
,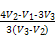 ӣ
ӣ

 Ņ»æĪŅ»Į·æĪŹ±“ļ±źĻµĮŠ“š°ø
Ņ»æĪŅ»Į·æĪŹ±“ļ±źĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

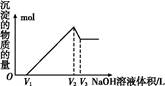
| ³ĮµķĪļ | Al(OH)3 | Fe(OH)3 | Mg(OH)2 |
| æŖŹ¼³ĮµķpH(Ąė×Ó³õŹ¼ÅضČ0.01 mol/L) | 4 | 2.3 | 10.4 |
| ĶźČ«³ĮµķpH(Ąė×ÓÅØ¶Č£¼10£5mol/L) | 5.2 | 4.1 | 12.4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
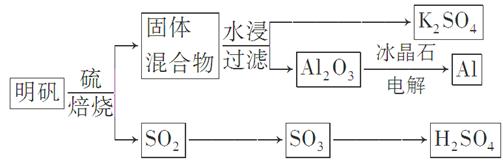 ±ŗÉÕĆ÷·ÆµÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ4KAl£ØSO4£©2”¤12H2O£«3S=2K2SO4£«2Al2O3£«9SO2”ü£«48H2O
±ŗÉÕĆ÷·ÆµÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ4KAl£ØSO4£©2”¤12H2O£«3S=2K2SO4£«2Al2O3£«9SO2”ü£«48H2O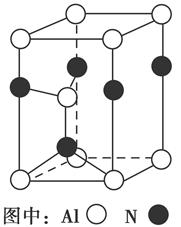
 2SO3£Øg£©””¦¤H1£½£197 kJ/mol£»
2SO3£Øg£©””¦¤H1£½£197 kJ/mol£»²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
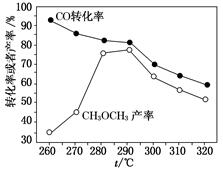
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®½šŹōĆ¾×Å»šŹ±æÉÓĆøɱłĆš»š |
| B£®¶žŃõ»Æ¹čæÉÓĆ×÷¹āµ¼ĻĖĪ¬ |
| C£®ĶłĀČĖ®ÖŠĶØČėSO2ŗó£¬ČÜŅŗµÄĘư׊ŌŌöĒæ |
| D£®ÓÉÓŚĀĮŌŚ³£ĪĀĻĀ²»ÓėŃõĘų·“Ó¦£¬ĖłŅŌĀĮÖĘĘ·¾ßÓŠæ¹øÆŹ“ŠŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®3£®2 g | B£®4£®0 g | C£®4£®2 g | D£®4£®6 g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®FeŌŚĀČĘųÖŠČ¼ÉÕÉś³ÉFeCl2 |
| B£®½«AlCl3ČÜŅŗÖšµĪµĪČėµ½NaOHČÜŅŗ£¬ĻČ²śÉś°×É«³Įµķ£¬×īŗó³ĮµķĻūŹ§ |
| C£®Ģś·ŪÖŠ»ģÓŠĀĮ·Ū¼ČæÉÓĆ¹żĮæµÄNaOHČÜŅŗ£¬Ņ²æÉŅŌÓĆ¹żĮæFeCl3³ä·Ö·“Ó¦¹żĀĖ³żČ„ |
| D£®³£ĪĀĻĀ£¬ĀĮÖĘĘ·ÓĆÅØĮņĖį»ņÅØĻõĖį“¦Ąķ¹ż£¬æÉÄĶøÆŹ“ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 2K2SO4+2Al2O3 +9SO2 +48H2O£¬ĻĀĮŠÅŠ¶ĻÕżČ·µÄŹĒ
2K2SO4+2Al2O3 +9SO2 +48H2O£¬ĻĀĮŠÅŠ¶ĻÕżČ·µÄŹĒ| A£®ŌŚ±ŗÉÕĆ÷·ÆµÄ·“Ó¦ÖŠ£¬»¹Ō¼ĮÓėŃõ»Æ¼ĮµÄĪļÖŹµÄĮæÖ®±ČŹĒ3£ŗ4 |
| B£®×īŗóµĆµ½µÄK2SO4ČÜŅŗ³ŹÖŠŠŌ£¬ĖłŅŌc(K+)=c(SO42-) |
| C£®±ŗÉÕ²śÉśµÄSO2æÉÓĆÓŚÖĘĮņĖį,±ŗÉÕ948 tĆ÷·Æ(M=" 474" g/mol)£¬ČōSO2µÄĄūÓĆĀŹĪŖ96%£¬æÉÉś²śÖŹĮæ·ÖŹżĪŖ98%µÄĮņĖį432 t |
| D£®¹¤ŅµÉĻŅ±Į¶Al2O3ÖʵĆAl£¬ŅŌAlŗĶNiO(OH)ĪŖµē¼«£¬NaOHČÜŅŗĪŖµē½āŅŗ×é³ÉŅ»ÖÖŠĀŠĶµē³Ų£¬·ÅµēŹ±NiO(OH)×Ŗ»ÆĪŖNi(OH)2£¬øƵē³Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ |
 NaAlO2£«3Ni(OH)2
NaAlO2£«3Ni(OH)2²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com