
��֪��P4(s)��6Cl2(g)��4PCl3(g) ��H��akJ·mol��1
P4(s)��10Cl2(g)��4PCl5(g) ��H�� bkJ·mol��1
P4������������ṹ��PCl5��P��Cl���ļ���ΪckJ·mol��1,PCl3��P��Cl���ļ���Ϊ1.2ckJ·mol��1
����������ȷ����( )
A��P��P���ļ��ܴ���P��Cl���ļ���
B������Cl2(g)��PCl3(g)��PCl5(s)�ķ�Ӧ�Ȧ�H
C��Cl��Cl���ļ��� kJ·mol��1
kJ·mol��1
D��P��P���ļ���Ϊ kJ·mol��1
kJ·mol��1
 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������8�����ʣ�������������գ�����ѡ©ѡ�����÷֣�
��Cu �����Ƶ���ˮ ��FeCl2��Һ �ܼ�ʯ��
�ݣ�NH4��2SO4���� �� KOH���� �� ϡ���� ��SO2
���ܵ������ �����ڵ���ʵ��� �����ڷǵ���ʵ��� ��
�Ƽ�ʯ�ҳ�����������������ܸ������� ����
A��H2 B��NH3 C��Cl2 D��O2 E��SO2
�������Һ�м���KBr��Һ����CCl4��ȡ��CCl4����غ�ɫ��д���÷�Ӧ���̵����ӷ���ʽ�������ת�Ʒ������Ŀ��
��д��ʵ���Ҽ�����е������ӵIJ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧ����ͼ�õ�ⷨ���ݵ缫���������ʵ���������֤�����ӵ�����ֵ����ʵ�鷽����Ҫ��Ϊ��

���õ�������Ȼ�ͭ��Һ����Ӧ������ͼ
���ڵ���ΪI A��ͨ��ʱ��Ϊt s��ȷ���ij�缫��������ͭ������Ϊm g���Իش�
(1)��Щ��������ȷ����˳��Ϊ(��ͼ�б�ע������������Ӣ����ĸ��ʾ����ͬ)
E�� �� �� �� ��F��ʵ����·�еĵ�������Ϊ____��____��____��____��____��____��
(2)д��B�缫�Ϸ�����Ӧ�����ӷ���ʽ__________________________________
________________��G�Թ��е���KI��Һ�仯������Ϊ________________����Ӧ�����ӷ���ʽ��____________________________________________________________��
(3)Ϊ��ȷ�ⶨ�缫������ͭ���������������ʵ�鲽����Ⱥ�˳����______________��
�ٳ������ǰ�缫����
�ڹ��µ���缫�ϵ�ͭ����ϴ
��������ˮ��ϴ����缫
�ܵ��º�ɵ缫�����
�ݵ��º�ɹ��µ�ͭ������
���ٴε��º�ɹ��µ�ͭ������
���ٴε��º�ɺ����������
(4)��֪���ӵĵ���Ϊ1.6��10��19���ء����г������ӵ������������ʽ��NA��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��ѧ���ܣ�Si—Si��M KJ/mol O=O��N KJ/mol Si—O��Q KJ/mol����Si (s) + O2 (g) ==SiO2 (s); ��H= �� ��
A��—��4Q—2M—N��KJ/mol B��—��4Q—M—2N��KJ/mol
C��—��2Q—M—2N��KJ/mol D��—��2Q—2M—N��KJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��״̬�£���̬���ӶϿ�l mol��ѧ�����ʱ��Ϊ���ʡ���֪H-H��H-O
��O-O���ļ��ʦ�H�ֱ�Ϊ436 kJ/mol��463 kJ/mol��495kJ/mol�������Ȼ�ѧ����ʽ��ȷ����
A��H2O (g)= H2(g)+ O2(g); ��H= -485 kJ/mol
O2(g); ��H= -485 kJ/mol
B��H2O (g)=H2(g)+ O2(g); ��H==+485 kJ/mol
O2(g); ��H==+485 kJ/mol
C��2H2(g)+ O2 (g)= 2H2O(g) ��H = +485 kJ/mol
D��2H2(g)+ O 2(g)=2H2O(g) ��H = -485 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�����ˮ���˵������ȷ����(����)
A������ˮ������ƻ��˴�ˮ�ĵ���ƽ��
B������ˮ�ⷴӦ������кͷ�Ӧ���淴Ӧ
C������ˮ��Ľ��ʹ��Һ��һ��������
D��Na2S��Һ��[Na��]��[S2��]��2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ����(����)
A��̼��ĵ��룺H2CO3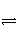
 2H����CO
2H����CO
B���������Ƽ���ˮ�У�2Na��2H2O===2Na����2OH����H2��
C������ˮ�⣺S2����2H2O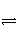
 H2S����2OH��
H2S����2OH��
D�����Ȼ�����Һ�м���������ռ���Һ��Al3����3OH��===Al(OH)3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̽��ʽ���ý�ѧ����ע��֪ʶ���γɹ��̶��ܺõ��������¿θĵ����ij����ѧϰ������
���Ƶ����ʡ�ʱ���ð�ͬѧ��������������ʵ�飺

��1���������
ʵ��һ��CO2�����ʵ�����CO2Ϊ���ܡ���𡱣�
��2�����������
��Na2O2��CO2�����˷�Ӧ��������Na2CO3���ɣ�
����ȼ�գ�˵���÷�Ӧ���ȣ�ʹ�¶ȴﵽ�������Ż�㣻
�۾���ȼ�գ���Ӧ�п����� ���ɡ�
��3����Ʋ�����ʵ�飬�۲졢���ͺͽ���
���������������±���
| ʵ�鲽�� | ʵ������ | ԭ��ͽ��� | ||
| �� | ��С��Ȫˮƿ�ռ���CO2������ƿ�м��벻ͬ��dz��ɫ��Na2O2;��ȼ�ŵ�ľ������ƿ�� | �ڢ�С�����������Na2O2 | ľ��δ��ȼ�������� | CO2��������Na2O2���㣩��ƿ��O2��CO2�࣬Na2O2ȫ�������˷�Ӧ |
| �ڢ�С������Na2O2�Զ�һЩ | ľ����ȼ�����ֹ����Ϊ��ɫ | |||
| �� | ��С������ִ���ƿ�ĵײ� | ƿ���� | ||
| �� | ��С�鶼��ȡ������ɫ�������Թ��У�����ϡ���Ტ���ϴ����ܵ�������������������ͨ�� | ��CO32-���ڣ�˵������������Na2CO3 |
��4���������
Na2O2��H2O��Ӧ��Ӧ����NaOH��O2���ɡ��������Na2O2�����ϵ�����ˮ����ҲӦ����ȼ�գ������ɵ���Һ�е����̪Ӧ�ñ��ɫ��
��5��ʵ�������
����С�ձ����ײ���ɰ���а���Na2O2����֬���ϵ�����ˮ�����������ݲ����������ȣ�����û��ȼ�գ���ԭ������� ��
�����е���Һ�����̪����Һ���ɫ�����ɫ��ʧ����ԭ������� ��
��6����������ʾ
����Na2O2��ע������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ں�����ߺ�DZˮͧ�п��ù���������Ϊ����������ѡ���ʵ��Ļ�ѧ�Լ���ʵ����Ʒ����ͼ�е�ʵ��װ�ý���ʵ�飬֤���������ƿ�����������

��1��A����ȡCO2��װ�ã�д��A�з�����Ӧ�Ļ�ѧ����ʽ��________________________________.
��2����д���пո�
| ���� | �����Լ� | ������Լ���Ŀ�� |
| B | ����NaHCO3��Һ | |
| C | ||
| D |
��3��д�����������������̼��Ӧ�Ļ�ѧ����ʽ��______________________
____________________________________________________________________.
��4���Թ�F���ռ����������һ��ʵ������ǣ�____________________________________
________________________________________________________________________.
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com