
| 8.1g |
| 27g/mol |
| 3g |
| 12g |
|



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
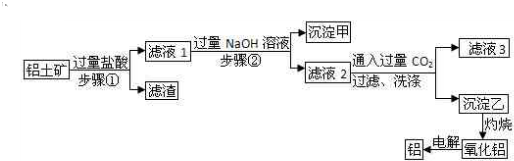
 �������и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������
�������и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������| a | b | c | |
| A | Al | AlCl3 | Al��OH��3 |
| B | HNO3 | NO | NO2 |
| C | Si | SiO2 | H2SiO3 |
| D | Na2O | Na2CO3 | NaHCO3 |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij�¶��£���ͬpH������ʹ�����Һ�ֱ��ˮϡ�ͣ�pH����Һ����仯��������ͼ��ʾ�������ж���ȷ���ǣ�������
ij�¶��£���ͬpH������ʹ�����Һ�ֱ��ˮϡ�ͣ�pH����Һ����仯��������ͼ��ʾ�������ж���ȷ���ǣ�������| A����ͬ���ʱ��c����Һ�кͼ����������a�� |
| B��b����Һ�ĵ����Ա�c����Һ�ĵ�����ǿ |
| C��b�����Ũ�ȴ���a�����Ũ�� |
| D����Ϊ����ϡ��ʱ��pH�仯���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����W���ڵ�ͬ����Ԫ�ص��ʵ���Ҫ��;���������� |
| B��X���ʲ������û���W���� |
| C��Ԫ��ԭ�Ӱ뾶�Ӵ�С��˳����X��Y��Z |
| D���ɷǽ�����ǿ����֪����������W�ĺ������Ʊ�Z�ĺ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| һ������ |
| ŨH2SO4 |
| �� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A��ԭ�Ӱ뾶��W��Y��Z��M��X |
| B��X��Y��Z ����Ԫ���γɵĻ������п��ܼ������Ӽ����й��ۼ� |
| C��W�ֱ���M��ZԪ���γɵĻ�����WM4��WZ2����ԭ�Ӿ��� |
| D��X�ֱ���Y��Z��M��W�γɵij����������У��ȶ�����õ���XM���е�X2Z��XM |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��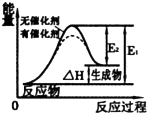 ͼ��ʾ�����ܸı仯ѧ��Ӧ���ʱ� |
B��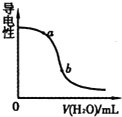 ͼ��ʾ��ˮ�м�ˮʱ��Һ�����Եı仯���������Һc��OH-����С��a��b |
C��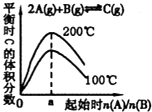 ��ͼ��֪��Ӧ2A��g��+B��g��?C��g���ġ�H��O���� a=2 |
D��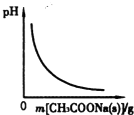 ͼ��ʾ��CH3COOH��Һ������CH3COONa�������ҺpH�ı仯��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com