
| A��������������С��9.7��ʱ������ijɷ�ΪH2S��H2 |
| B��ԭ������е�ZnΪ6.5�� |
| C������������������9.7��ʱ������ijɷֽ�ΪH2S |
| D����ȼ�����ʵ���H2S��O2�Ļ�����壬���ɵĺ�����Ϊ������CS2�ĵ��� |


 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������KSCN��Һһ�������ɫ |
| B����Һ��һ����Fe2+ |
| C����Һ��һ������Cu2+ |
| D��ʣ�������һ����Cu |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Һ̬����Ǧ�к������ӽ� | B��20.7gǦ������ϡ���ᷴӦ����2.24L���� |
| C��������Ǧ�λ������ж� | D��Һ̬����Ǧ���������е�������ö��γ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ���ᷴӦ)��
���ᷴӦ)��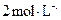 ���ᡢ5%NaOH��Һ������
���ᡢ5%NaOH��Һ������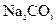 ��Һ������
��Һ������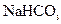 ��Һ������ˮ��
��Һ������ˮ��
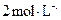 ���ᣬ�������ӡ���B��E�о����뱥��
���ᣬ�������ӡ���B��E�о����뱥��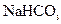 ��Һ,��ͼ��ʾ�����������ܶ�����
��Һ,��ͼ��ʾ�����������ܶ����� ����ͬһˮƽ���ϣ�������ʱ�ҹܵ�Һ�������ܵ�Һ�棬Ӧ���еIJ�����
����ͬһˮƽ���ϣ�������ʱ�ҹܵ�Һ�������ܵ�Һ�棬Ӧ���еIJ�����  ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
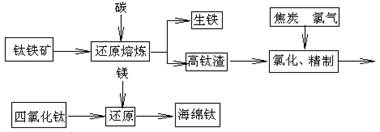
 TiO2+Fe+CO��,��FeTiO3�������������У��ѵĻ��ϼ�Ϊ ��
TiO2+Fe+CO��,��FeTiO3�������������У��ѵĻ��ϼ�Ϊ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������� | B���������� | C���������� | D����ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NO | B��NO2 | C��NO��N2O | D��N2O��NH4NO3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com