
| 5-1”Į3 |
| 2 |


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ”¾»Æѧ--Ń”ŠŽ3ĪļÖŹ½į¹¹ÓėŠŌÖŹ”æ
”¾»Æѧ--Ń”ŠŽ3ĪļÖŹ½į¹¹ÓėŠŌÖŹ”æ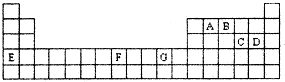
 øĆÅäĄė×ÓÖŠŗ¬ÓŠµÄ»Æѧ¼üĄąŠĶÓŠ
øĆÅäĄė×ÓÖŠŗ¬ÓŠµÄ»Æѧ¼üĄąŠĶÓŠ| 1 | 8 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
(A)”¾ĪļÖŹ½į¹¹ÓėŠŌÖŹ”æ
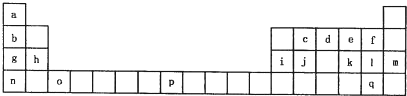
ĻĀ±ķŹĒŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö”£±ķÖŠĖłĮŠµÄ×ÖÄø·Ö±š“ś±ķijŅ»ÖÖ»ÆѧŌŖĖŲ”£

(1)T3+µÄŗĖĶāµē×ÓÅŲ¼Ź½ŹĒ____________”£
(2)Q”¢R”¢MµÄµŚŅ»µēĄėÄÜÓɓ󵽊”µÄĖ³ŠņŹĒ___________________(ÓĆŌŖĖŲ·ūŗűķŹ¾)”£
(3)ĻĀĮŠÓŠ¹ŲÉĻŹöŌŖĖŲµÄĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ______________________(ĢīŠņŗÅ)”£
¢ŁGµ„ÖŹµÄČŪµćøßÓŚJµ„ÖŹ£¬ŹĒŅņĪŖGµ„ÖŹµÄ½šŹō¼ü½ĻĒæ
¢ŚJ±ČX»īĘĆ£¬ĖłŅŌJæÉŅŌŌŚČÜŅŗÖŠÖĆ»»³öX
¢Ū½«J
¢ÜRE3·ŠµćøßÓŚQE4£¬Ö÷ŅŖŹĒŅņĪŖĒ°ÕßĻą¶Ō·Ö×ÓÖŹĮæ½Ļ“ó
¢ŻŅ»øöQ2E4·Ö×ÓÖŠŗ¬ÓŠĪåøö¦Ņ¼üŗĶŅ»øö¦Š¼ü
(4)¼ÓÄĆ“óĢģĪÄĢØŌŚĢ«æÕ·¢ĻÖĮĖEQ9R£¬ŅŃÖŖ·Ö×ÓÖŠĖłÓŠŌ×Ó¾łŠĪ³É8µē×Ó»ņ2µē×ÓĪČ¶Ø½į¹¹£¬ŹĒÖ±ĻߊĪ·Ö×Ó£¬²»“ęŌŚÅäĪ»¼ü”£Š“³öĘä½į¹¹Ź½£ŗ_________________”£
(5)GÓėRµ„ÖŹÖ±½Ó»ÆŗĻÉś³ÉŅ»ÖÖĄė×Ó»ÆŗĻĪļG3R”£øĆ¾§Ģå¾ßÓŠĄąĖĘŹÆÄ«µÄ²ćד½į¹¹”£Ćæ²ćÖŠ£¬GŌ×Ó¹¹³ÉĘ½ĆęĮł±ßŠĪ£¬ĆæøöĮł±ßŠĪµÄÖŠŠÄÓŠŅ»øöRŌ×Ó”£²ćÓė²ćÖ®¼ä»¹¼ŠŌÓŅ»¶ØŹżĮæµÄŌ×Ó”£ĒėĪŹÕāŠ©¼ŠŌÓµÄŌ×ÓÓ¦øĆŹĒ____________(ĢīG»ņRµÄŌŖĖŲ·ūŗÅ)”£

(B)”¾ŹµŃé»Æѧ”æ
Ä³×ŹĮĻĻŌŹ¾£¬ÄÜŹ¹Ė«ŃõĖ®·Ö½āµÄ“߻ƼĮÓŠŗܶąÖÖ£¬ÉśĪļ“߻ƼĮ(ČēÖķøĪ)”¢Ąė×ÓŠĶ“߻ƼĮ(ČēFeCl3)ŗĶ¹ĢĢå“߻ƼĮ(ČēMnO2)µČ¶¼ŹĒ½ĻŗĆµÄ“ß»Æ¼Į”£Ä³ŹµŃ銔×éĶعż²ā¶ØĖ«ŃõĖ®·Ö½ā²śÉśµÄO2µÄŃ¹Ē棬Ģ½¾æ·Ö½ā¹żŃõ»ÆĒāµÄ×ī¼Ń“߻ƼĮŅŌ¼°Ģ½¾æ×ī¼Ń“߻ƼĮŗĻŹŹµÄ“ß»ÆĢõ¼ž”£
(Ņ»)Ģ½¾æŅ»£ŗ
ŹµŃé²½Öč
(1)Ķł×¶ŠĪĘæÖŠ¼ÓČė50 mL 1.5£„µÄĖ«ŃõĖ®
(2)·Ö±šĶł×¶ŠĪĘæÖŠ¼Ó
(3)²É¼ÆŗĶ¼ĒĀ¼Źż¾Ż”£
(4)ÕūĄķŹż¾ŻµĆ³öĻĀ±ķ
²»Ķ¬“߻ƼĮ”°Ń¹Ēæ¶ŌŹ±¼äŠ±ĀŹ”±µÄ±Č½Ļ
“߻ƼĮ | ÖķøĪ | ĀķĮåŹķ | ĀČ»ÆĶ | ĀČ»ÆĢś | Ńõ»ÆĶ | ¶žŃõ»ÆĆĢ |
Ń¹Ēæ¶ŌŹ±¼äµÄŠ±ĀŹ | 0.191 87 | 0.002 42 | 0.007 93 | 0.030 5 | 0.015 47 | 1.833 6 |
¢ŁøĆ”°Ģ½¾æŅ»”±ŹµŃéµÄĆū³ĘŹĒ_____________________________________________________”£
¢ŚøĆŹµŃéĖłµĆ³öµÄ½įĀŪŹĒ_______________________________________________________”£
(¶ž)Ģ½¾æ¶ž£ŗ¶žŃõ»ÆĆĢ“߻ƵÄ×ī¼Ń“ß»ÆĢõ¼ž
øĆŹµŃ銔×éµÄĶ¬Ń§ŌŚ½ųŠŠĢ½¾æ¶žµÄŹµŃ鏱£¬µĆµ½ĮĖŅ»ĻµĮŠµÄĶ¼±ķŗĶŹż¾Ż”£²Īæ“ĻĀĶ¼ŗĶ±ķøń·Ö±š»Ų“šĻą¹ŲĪŹĢā”£

3%µÄĖ«ŃõĖ®Óė²»Ķ¬ÓĆĮ涞Ńõ»ÆĆĢµÄŃ¹Į¦”ŖŹ±¼äĶ¼
±ķ£ŗ²»Ķ¬ÅØ¶ČµÄĖ«ŃõĖ®ŌŚ²»Ķ¬ÓĆĮæµÄ¶žŃõ»ÆĆĢ×÷ÓĆĻĀŹÕ¼ÆĻąĶ¬×“æöĻĀĶ¬Ģå»żO2ĖłŠčŹ±¼ä
MnO2 Ź±¼ä H2O2 | |||
1.5£„ | 223 s | 67 s | 56 s |
3.0£„ | 308 s | 109 s | 98 s |
4.5£„ | 395 s | 149 s | 116 s |
·ÖĪöĶ¼”¢±ķÖŠŹż¾ŻĪŅĆĒæÉŅŌµĆ³ö£ŗ
¢ŪĶ¬ÅØ¶ČµÄĖ«ŃõĖ®µÄ·Ö½āĖŁĀŹĖę×ŶžŃõ»ÆĆĢÓĆĮæµÄŌö¼Ó¶ų_________________£¬Ņņ¶ų·“Ó¦Ź±¼ä_______________”£
¢ÜČē¹ū“ÓŹµŃé½į¹ūŗĶ½ŚŹ”Ņ©Ę·µÄ½Ē¶Č×ŪŗĻ·ÖĪö£¬ÄćČĻĪŖµ±ĪŅĆĒŃ”ÓĆ3.0%µÄĖ«ŃõĖ®£¬¼ÓČė___________ gµÄ¶žŃõ»ÆĆĢÄÜŹ¹ŹµŃ銧¹ū×ī¼Ń”£ÄćÅŠ¶ĻµÄĄķÓÉŹĒ______________________”£
¢ŻøĆŠ”×éµÄijĶ¬Ń§Ķعż·ÖĪöŹż¾ŻµĆ³öĮĖµ±“߻ƼĮÓĆĮæĻąĶ¬Ź±Ė«ŃõĖ®µÄÅضČŌ½Š”·“Ó¦ĖŁĀŹŌ½æģµÄ½įĀŪ£¬ÄćČĻĪŖŹĒ·ńÕżČ·____________£¬ÄćµÄĄķÓÉŹĒ________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗÉĀĪ÷Ź”Ī÷°²ŹŠĪ劣2010½ģµŚ¶ž“ĪÄ£Äāæ¼ŹŌ£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
”¾»Æѧ”ŖĪļÖŹ½į¹¹ÓėŠŌÖŹ”æ£Ø15·Ö£©
ĻĀ±ķÖŠŹµĻߏĒŌŖĖŲÖÜĘŚ±ķµÄ²æ·Ö±ß½ē£¬ĘäÖŠÉĻ±ß½ē²¢Ī“ÓĆŹµĻß±ź³ö”£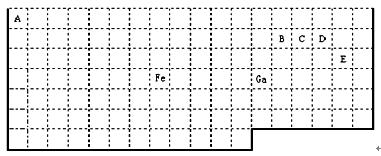
øł¾ŻŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÖÜĘŚ±ķÖŠ±ČGaÖŹ×ÓŹżÉŁ2µÄ»łĢ¬Ō×Ó¼Ūµē×ÓÅŲ¼Ź½ĪŖ ”£
£Ø2£©FeŌŖĖŲĪ»ÓŚÖÜĘŚ±ķµÄ ·ÖĒų£»FeÓėCOŅ׊Ī³ÉÅäŗĻĪļFe(CO)5£¬ŌŚFe(CO)5ÖŠĢśµÄ»ÆŗĻ¼ŪĪŖ__________£»ŅŃÖŖ£ŗŌ×ÓŹżÄæŗĶµē×Ó×ÜŹż£Ø»ņ¼Ūµē×Ó×ÜŹż£©ĻąĶ¬µÄĪ¢Į£»„ĪŖµČµē×ÓĢ壬µČµē×ÓĢå¾ßÓŠĻąĖĘµÄ½į¹¹ĢŲÕ÷”£ÓėCO·Ö×Ó»„ĪŖµČµē×ÓĢåµÄ·Ö×ÓŗĶĄė×Ó·Ö±šĪŖ””””””””____ŗĶ””””””_____£ØĢī»ÆѧŹ½£©£¬COµÄ½į¹¹Ź½ĪŖ”” ”£
£Ø3£©ŌŚCH4”¢CO¼°CH3OHÖŠ£¬Ģ¼Ō×Ó²ÉČ”sp3ŌӻƵķÖ×ÓĪŖ”””””””””””””””””””£
£Ø4£©øł¾ŻVSEPRĄķĀŪŌ¤²āED4- Ąė×ÓµÄæռ乹ŠĶĪŖ______________ŠĶ”£B”¢C”¢D¼°EŌ×ÓĻą»„»ÆŗĻŠĪ³ÉµÄ·Ö×ÓÖŠ£¬ĖłÓŠŌ×Ó¶¼Āś×ć×īĶā²ć8µē×ÓĪČ¶Ø½į¹¹µÄµē×ÓŹ½ĪŖ£ŗ__________________________________(Š“2ÖÖ) ”£
£Ø5£©BÓėDŠĪ³ÉµÄĪČ¶Ø»ÆŗĻĪļĪŖ___________·Ö×Ó£ØĢī”°¼«ŠŌ”±”°·Ē¼«ŠŌ”±£©£¬Ęä¹ĢĢ¬ĪŖ ________¾§Ģ唣
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģŗŚĮś½Ź”¹žĮłÖŠøßČżµŚČż“ĪÄ£Äāæ¼ŹŌĄķ×Ū»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø15·Ö£©”¾»Æѧ---Ń”ŠŽÄ£æé£ŗĪļÖŹ½į¹¹ÓėŠŌÖŹ”æ
ĻĀ±ķĪŖÖÜĘŚ±ķµÄŅ»²æ·Ö£¬ĘäÖŠµÄ±ąŗÅ“ś±ķ¶ŌÓ¦µÄŌŖĖŲ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©±ķÖŠŹōÓŚdĒųµÄŌŖĖŲŹĒ £ØĢī±ąŗÅ£©”£
£Ø2£©ŌŖĖŲ¢ŽŠĪ³ÉµÄ×īøß¼Ūŗ¬ŃõĖįøłµÄĮ¢Ģå¹¹ŠĶŹĒ________£¬ĘäÖŠŠÄŌ×ÓµÄŌӻƹģµĄĄąŠĶŹĒ_______”£
£Ø3£©ŌŖĖŲ¢ŚµÄŅ»ÖÖĒā»ÆĪļŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬³£°ŃøĆĒā»ÆĪļµÄ²śĮæ×÷ĪŖŗāĮæŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½µÄ±źÖ¾”£ÓŠ¹ŲøĆĒā»ÆĪļ·Ö×ÓµÄĖµ·ØÕżČ·µÄŹĒ ”£
| A£®·Ö×ÓÖŠŗ¬ÓŠĒā¼ü | B£®ŹōÓŚ·Ē¼«ŠŌ·Ö×Ó |
| C£®ŗ¬ÓŠ4øö¦Ņ¼üŗĶ1øö¦Š¼ü | D£®øĆĒā»ÆĪļ·Ö×ÓÖŠ£¬¢ŚŌ×Ó²ÉÓĆsp2ŌÓ»Æ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğŗŚĮś½Ź”øßČżµŚČż“ĪÄ£Äāæ¼ŹŌĄķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø15·Ö£©”¾»Æѧ---Ń”ŠŽÄ£æé£ŗĪļÖŹ½į¹¹ÓėŠŌÖŹ”æ
ĻĀ±ķĪŖÖÜĘŚ±ķµÄŅ»²æ·Ö£¬ĘäÖŠµÄ±ąŗÅ“ś±ķ¶ŌÓ¦µÄŌŖĖŲ”£

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©±ķÖŠŹōÓŚdĒųµÄŌŖĖŲŹĒ £ØĢī±ąŗÅ£©”£
£Ø2£©ŌŖĖŲ¢ŽŠĪ³ÉµÄ×īøß¼Ūŗ¬ŃõĖįøłµÄĮ¢Ģå¹¹ŠĶŹĒ________£¬ĘäÖŠŠÄŌ×ÓµÄŌӻƹģµĄĄąŠĶŹĒ_______”£
£Ø3£©ŌŖĖŲ¢ŚµÄŅ»ÖÖĒā»ÆĪļŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬³£°ŃøĆĒā»ÆĪļµÄ²śĮæ×÷ĪŖŗāĮæŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½µÄ±źÖ¾”£ÓŠ¹ŲøĆĒā»ÆĪļ·Ö×ÓµÄĖµ·ØÕżČ·µÄŹĒ ”£
A£®·Ö×ÓÖŠŗ¬ÓŠĒā¼ü B£®ŹōÓŚ·Ē¼«ŠŌ·Ö×Ó
C£®ŗ¬ÓŠ4øö¦Ņ¼üŗĶ1øö¦Š¼ü D£®øĆĒā»ÆĪļ·Ö×ÓÖŠ£¬¢ŚŌ×Ó²ÉÓĆsp2ŌÓ»Æ
£Ø4£©Ä³ŌŖĖŲµÄĢŲÕ÷µē×ÓÅŲ¼Ź½ĪŖnsnnpn+1£¬øĆŌŖĖŲŌ×ÓµÄŗĖĶā×īĶā²ćµē×ӵĹĀ¶Ōµē×ÓŹżĪŖ £»øĆŌŖĖŲÓėŌŖĖŲ¢ŁŠĪ³ÉµÄ·Ö×ÓX¹¹ŠĪĪŖ £»XŌŚ¢ŁÓė¢ŪŠĪ³ÉµÄ·Ö×ÓYÖŠµÄČܽā¶ČŗÜ“ó£¬ĘäÖ÷ŅŖŌŅņŹĒ ”£
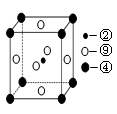
£Ø5£©æĘѧ·¢ĻÖ£¬¢Ś”¢¢Ü”¢¢įČżÖÖŌŖĖŲµÄŌ×ÓŠĪ³ÉµÄ¾§Ģå¾ßÓŠ³¬µ¼ŠŌ£¬Ę侧°ūµÄ½į¹¹ĢŲµćČēĶ¼£ØĶ¼ÖŠ¢Ś”¢¢Ü”¢¢į·Ö±šĪ»ÓŚ¾§°ūµÄĢåŠÄ”¢¶„µć”¢ĆęŠÄ£©£¬ŌņøĆ»ÆŗĻĪļµÄ»ÆѧŹ½ĪŖ £ØÓƶŌÓ¦µÄŌŖĖŲ·ūŗűķŹ¾£©”£
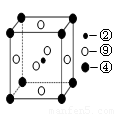
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com