
ĻĀĮŠ³£¼ūŹµŃéµÄĻÖĻó»ņ±ķŹöÕżČ·µÄŹĒ(””””)”£
A£®ĻņijČÜŅŗÖŠ¼ÓČė2µĪKSCNČÜŅŗ£¬ČÜŅŗ²»ĻŌŗģÉ«£¬ŌŁĻņČÜŅŗÖŠ¼ÓČė¼øµĪŠĀÖʵÄĀČĖ®£¬ČÜŅŗ±äĪŖŗģÉ«£¬øĆČÜŅŗÖŠŅ»¶Øŗ¬ÓŠFe2£«
B£®ĪŖ¼ģŃéŗģש֊ĢśµÄ¼ŪĢ¬£¬Ļņŗģש·ŪÄ©ÖŠ¼ÓČėŃĪĖį£¬³ä·Ö·“Ó¦ŗóČ”ÉĻ²ćĒåŅŗÓŚŹŌ¹ÜÖŠ£¬µĪ¼ÓKSCNČÜŅŗ2”«3µĪ£¬ČÜŅŗ±äĪŖŗģÉ«£¬ĖµĆ÷ŗģש֊ֻӊȿŃõ»Æ¶žĢś
C£®ÖʱøĒāŃõ»ÆŃĒĢśŹ±£¬ĻņĮņĖįŃĒĢśČÜŅŗÖŠµĪ¼ÓĒāŃõ»ÆÄĘČÜŅŗ£¬±ß¼Ó±ß½Į°č£¬¼“æÉÖʵư×É«µÄĒāŃõ»ÆŃĒĢś
D£®½«(NH4)2Fe(SO4)2”¤6H2OŹŌŃłČÜÓŚĻ”ĻõĖįÖŠ£¬µĪ¼ÓKSCNČÜŅŗ£¬³öĻÖŃŖŗģÉ«£¬ĖµĆ÷¼ģŃéĒ°øĆŹŌŃłŅѱäÖŹ

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
NaClOŗĶKAl(SO4)2¶¼ŹĒÖŲŅŖµÄ»Æ¹¤²śĘ·£¬¾łæÉÓ¦ÓĆÓŚŌģÖ½Ņµ”£
(1)NaClOČÜŅŗpH£¾7£¬ŌŅņŹĒ__________________(ÓĆĄė×Ó·“Ó¦·½³ĢŹ½±ķŹ¾)”£
(2)øł¾ŻNaClOµÄŠŌÖŹĶĘ²ā£¬ŌŚÖ½½¬ÖŠ¼ÓČėNaClOČÜŅŗµÄÄæµÄŹĒ____________”£
(3)ŌŚ1 mol”¤L-1µÄKAl(SO4)2ČÜŅŗÖŠĄė×ÓÅضČÓɓ󵽊”µÄĖ³ŠņŹĒ____________”£
(4)ijŠ”×éĶ¬Ń§ÓĆĻĀĶ¼ĖłŹ¾×°ÖĆĢ½¾æNaClOŗĶKAl(SO4)2±„ŗĶČÜŅŗ»ģŗĻ·“Ó¦µÄŹµŃ锣
¢Ł“ņæŖ»īČūĻņÉÕĘæÖŠ¼ÓČė±„ŗĶKAl(SO 4)2ČÜŅŗ£¬²śÉś“óĮæµÄ°×É«½ŗד³Įµķ”£·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ_____________________________________________________”£
4)2ČÜŅŗ£¬²śÉś“óĮæµÄ°×É«½ŗד³Įµķ”£·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ_____________________________________________________”£
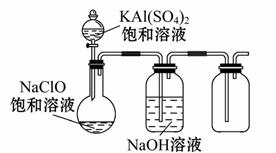
¢Ś½«ÉÕĘæÖŠµÄ»ģŗĻŅŗŌŚŃō¹āĻĀÕÕÉ䣬²»¾ĆÉÕĘæÖŠÓŠ»ĘĀĢÉ«ĘųĢå²śÉś”£³ä·Ö·“Ó¦ŗó¼ÆĘųĘæÖŠĘųĢåÄÜŹ¹“ų»šŠĒµÄľĢõø“Č¼”£Š“³öŌŚ¹āÕÕĻĀ»ģŗĻŅŗÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½______________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ņ±Į¶ĶæóŹÆĖł»ńµĆµÄĶĶس£ŗ¬ÓŠŠæ”¢Ģś”¢Äų”¢Ņų”¢½šŗĶ²¬µČĪ¢ĮæŌÓÖŹ£¬Ė×³Ę“ÖĶ”£¹¤ŅµÉĻĶس£Ķعżµē½ā·Ø³żČ„ÕāŠ©ŌÓÖŹÖĘµĆ¾«Ķ£¬ŅŌĢįøßĶµÄŹ¹ÓĆ¼ŪÖµ£¬Ą©“óĶµÄÓ¦ÓĆ·¶Ī§”£(¼øÖÖ½šŹōµÄĻą¶ŌŌ×ÓÖŹĮæŹĒ£ŗFe£56£¬Ni£59£¬Cu£64£¬Zn£65£¬Ag£108£¬Au£197”£)
ĒėĶź³ÉĻĀĮŠĪŹĢā£ŗ
(1)Ņ»°ćĄ“Ėµ£¬µē½ā¾«Į¶ĶµÄ³õŹ¼µē½āÖŹČÜŅŗĄļµÄŃōĄė×ÓŹĒ__________£¬Š“³öĶµÄµē½ā¾«Į¶¹ż³ĢÖŠµÄŅõ¼«·“Ó¦Ź½________________________________”£
(2)Čē¹ū×ŖŅĘ0.020 mol e££¬ĻĀĮŠĖµ·ØÖŠŅ»¶ØÕżČ·µÄŹĒ________”£
¢ŁŅõ¼«ÖŹĮæŌö¼Ó0.64 g””¢ŚŃō¼«ÖŹĮæ¼õÉŁ0.64 g
¢Ūµē½āÖŹČÜŅŗµÄÖŹĮæ±£³Ö²»±ä””¢Üµē½āÖŹČÜŅŗµÄĪĀ¶Č±£³Ö²»±ä
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ļņ200 mLĻ”ĻõĖįČÜŅŗÖŠ¼ÓČė11.2 gĢś·Ū£¬³ä·Ö·“Ó¦ŗó£¬Ģś·ŪČ«²æČܽā²¢·Å³öNOĘųĢ壬ČÜŅŗÖŹĮæŌö¼Ó7.0 g£¬ŌņĖłµĆČÜŅŗÖŠFe3£«µÄĪļÖŹµÄĮæÅضČŌ¼ĪŖ
(””””)”£
A£®0.1 mol/L B£®0.2 mol/L
C£®0.3 mo/L D£®0.4 mo/L
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓŠ¹Ų¹č¼°Ęä»ÆŗĻĪļµÄĖµ·ØÕżČ·µÄŹĒ(””””)”£
A£®µ„ÖŹ¹č³£ÓĆ×÷°ėµ¼ĢåŗĶ¹āµ¼ĻĖĪ¬²ÄĮĻ
B£®¹čŌŚ×ŌČ»½ēÖŠÖ»ŅŌ»ÆŗĻĢ¬µÄŠĪŹ½“ęŌŚ
C£®SiO2Óė“æ¼īøßĪĀÉś³ÉCO2£¬ĖµĆ÷¹čĖįµÄĖįŠŌ±ČĢ¼ĖįĒæ
D£®SiO2ŹĒ·Ē½šŹōŃõ»ÆĪļ£¬Ėü²»ÓėČĪŗĪĖį·“Ó¦
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
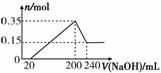
Ä³ŃŠ¾æŠ”×齫Ņ»¶ØÖŹĮæµÄĆ¾ĀĮŗĻ½š²ÄĮĻĶ¶Čė200 mLĮņĖįÖŠ£¬¹ĢĢåČ«²æČܽāŗó£¬ĻņĖłµĆČÜŅŗÖŠ¼ÓČėNaOHČÜŅŗ£¬Éś³É³ĮµķµÄĪļÖŹµÄĮæn(mol)Óė¼ÓČėNaOHČÜŅŗµÄĢå»żV(mL)µÄ¹ŲĻµČēĶ¼ĖłŹ¾”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)øĆŗĻ½š²ÄĮĻÖŠ£¬Ć¾”¢ĀĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ______”£
(2)ĮņĖįµÄĪļÖŹµÄĮæÅضČĪŖ______”£
(3)ĮķČ”ĻąĶ¬ÖŹĮæµÄøĆŗĻ½š²ÄĮĻÓė7.8 g Na2O2Ņ»ĘšĶ¶Čė×ćĮæµÄH2OÖŠ£¬×īÖÕ²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¶ŌÓŚ·“Ó¦£ŗM£«NØD”śP£¬Čē¹ūĪĀ¶ČĆæÉżøß10”ę£¬»Æѧ·“Ó¦ĖŁĀŹĢįøßµ½ŌĄ“µÄ3±¶£¬ŌŚ10”ꏱĶź³É·“Ó¦µÄ10%ŠčŅŖ81 min£¬½«ĪĀ¶ČĢįøßµ½30”ꏱ£¬Ķź³É·“Ó¦µÄ10%ŠčŅŖµÄŹ±¼äĪŖ(””””)
A£®9 min B£®27 min C£®13.5 min D£®3 min
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
1£®ĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ(””””)””””””””””””””””””””””””””””””””
¢Ł»ī»Æ·Ö×Ó¼äµÄÅöײŅ»¶ØÄÜ·¢Éś»Æѧ·“Ó¦””¢ŚĘÕĶØ·Ö×Ó¼äµÄÅöײӊŹ±Ņ²ÄÜ·¢Éś»Æѧ·“Ó¦””¢Ū»ī»Æ·Ö×Ó±ČĘÕĶØ·Ö×Ó¾ßÓŠ½ĻøßµÄÄÜĮæ””¢Ü»Æѧ·“Ó¦µÄŹµÖŹŹĒŌ×ÓµÄÖŲŠĀ×éŗĻ ¢Ż»Æѧ·“Ó¦µÄŹµÖŹŹĒ¾É»Æѧ¼üµÄ¶ĻĮŃŗĶŠĀ»Æѧ¼üµÄŠĪ³É¹ż³Ģ ¢Ž»Æѧ·“Ó¦µÄŹµÖŹŹĒ»ī»Æ·Ö×ÓÓŠŗĻŹŹČ”ĻņµÄÓŠŠ§Åöײ
A£®¢Ł¢Ū¢Ü¢Ż B£®¢Ś¢Ū¢Ž C£®¢Ü¢Ż¢Ž D£®¢Ś¢Ü¢Ż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Na2SO3ŌŚæÕĘųÖŠŅ×±»Ńõ»Æ¶ų±äÖŹ”£Ä³Ķ¬Ń§ĪŖÖ¤Ć÷Na2SO3ÓŠ»¹ŌŠŌ£¬“ÓŅ»Ęæ³¤ĘŚ“ę·ÅµÄNa2SO3¹ĢĢåÖŠČ”³öÉŁĮæČÜÓŚĖ®£¬µĪČėŅ»¶ØĮæµÄÉÕ¼īČÜŅŗŗĶÉŁŠķäåĖ®£¬Õńµ“ŗóČÜŅŗ±äĪŖĪŽÉ«”£
(1)ŌŚ¼īŠŌČÜŅŗÖŠ£¬Br2ÓėNa2SO3·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ__________________________________________________________”£
(2)·“Ó¦ŗó£¬ČÜŅŗŗ¬ÓŠSO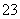 ”¢SO
”¢SO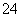 ”¢Br£”¢OH£µČŅõĄė×Ó£¬ĻĀ±ķŹĒijĶ¬Ń§¼ų¶ØĘäÖŠSO
”¢Br£”¢OH£µČŅõĄė×Ó£¬ĻĀ±ķŹĒijĶ¬Ń§¼ų¶ØĘäÖŠSO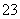 ”¢SO
”¢SO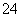 ŗĶBr£µÄŹµŃé±Øøę£¬ĒėĶź³ÉĪ“ĢīĶźµÄ²æ·Ö”£
ŗĶBr£µÄŹµŃé±Øøę£¬ĒėĶź³ÉĪ“ĢīĶźµÄ²æ·Ö”£
ĻŽŃ”ŹŌ¼Į£ŗ2 mol”¤L£1ŃĪĖį£»1 mol”¤L£1H2SO4ČÜŅŗ£»1 mol”¤L£1BaCl2ČÜŅŗ£»1 mol”¤L£1Ba(NO3)2ČÜŅŗ£»CCl4”¢ŠĀÖʱ„ŗĶĀČĖ®”¢Ę·ŗģČÜŅŗ”£
| ±ąŗÅ | ŹµŃé²Ł×÷ | Ō¤ĘŚĻÖĻóŗĶ½įĀŪ |
| ²½Öč¢Ł | ȔɣĮæ“ż²āŅŗÓŚŹŌ¹ÜÖŠ£¬¼ÓČė¹żĮæµÄ2 mol”¤L£1ŃĪĖį£¬ŌŁµĪ¼ÓŹŹĮæ1 mol”¤L£1BaCl2ČÜŅŗ | ÓŠ°×É«³ĮµķÉś³É£¬Ö¤Ć÷“ż²āŅŗÖŠŗ¬SO |
| ²½Öč¢Ś | ||
| ²½Öč¢Ū |
(3)ĪŖ²ā¶ØÉĻŹöѳʷµÄ“æ¶Č£¬øĆĶ¬Ń§Č”10.0 gŹŌŃł£¬Åä³É250 mLČÜŅŗ£¬Č”25.00 mLĖłÅäČÜŅŗ£¬ÓĆ0.10 mol”¤L£1µÄĖįŠŌKMnO4ČÜŅŗµĪ¶ØÖĮÖÕµć”£Ąė×Ó·“Ó¦ĪŖ2MnO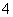 £«5SO
£«5SO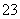 £«6H£«===2Mn2£«£«5SO
£«6H£«===2Mn2£«£«5SO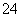 £«3H2O£¬ÖŲø“²Ł×÷Čż“Ī£¬Ćæ“ĪĻūŗÄ0.10 mol”¤L£1 KMnO4ČÜŅŗµÄĢå»ż·Ö±šĪŖ20.02 mL”¢20.00 mLŗĶ19.98 mL”£
£«3H2O£¬ÖŲø“²Ł×÷Čż“Ī£¬Ćæ“ĪĻūŗÄ0.10 mol”¤L£1 KMnO4ČÜŅŗµÄĢå»ż·Ö±šĪŖ20.02 mL”¢20.00 mLŗĶ19.98 mL”£
¢ŁøĆŹŌŃłÖŠNa2SO3µÄÖŹĮæ·ÖŹżĪŖ________(½į¹ū±£Įō3Ī»ÓŠŠ§Źż×Ö)£»
¢Ś²Ł×÷Ź±£¬ČōĪ“ÓĆ0.10 mol”¤L£1µÄĖįŠŌKMnO4ČÜŅŗČóĻ“µĪ¶Ø¹Ü£¬»įµ¼ÖĀ²ā¶Ø½į¹ū________(Ģī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°Ć»ÓŠÓ°Ļģ”±)”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com