
�Ի�����Ϊԭ����������Ĺ�������ͼ���£�

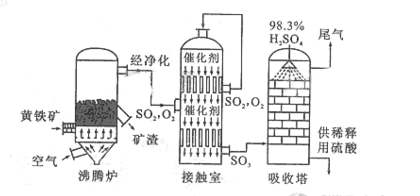
 ��1����ȼ�ջ�����Ļ�ѧ����ʽ��������www.ks5.u.com
��1����ȼ�ջ�����Ļ�ѧ����ʽ��������www.ks5.u.com
4
+11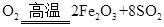
 ��2���Ӵ����з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2���Ӵ����з�����Ӧ�Ļ�ѧ����ʽ�� ��
��3�����ݹ�������ͼ�ж�����˵����ȷ���ǣ�ѡ�������ĸ�� ��
 a. Ϊʹ��������ȼ�գ��轫�����
a. Ϊʹ��������ȼ�գ��轫�����
b. ������������� ��ת����
��ת����
 c. ʹ�ô��������
c. ʹ�ô�������� �ķ�Ӧ���ʺ�ת����
�ķ�Ӧ���ʺ�ת����
d. ����¯�ų��Ŀ����ɹ�����
 ��4��ÿ160g
��4��ÿ160g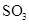 ������
������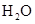 ���Ϸų�260.6kJ���������÷�Ӧ���Ȼ�ѧ������
��
���Ϸų�260.6kJ���������÷�Ӧ���Ȼ�ѧ������
��
��5���������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�����õ��ϸ�Ũ�ȵ�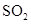 ����Ρ�
�����
 ��
��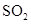 �ȿ���Ϊ���������ԭ��ѭ�������ã�Ҳ�����ڹ�ҵ������������ճ�ʪ�����е�
�ȿ���Ϊ���������ԭ��ѭ�������ã�Ҳ�����ڹ�ҵ������������ճ�ʪ�����е�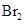 ��
��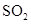 ����
����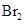 �����ӷ���ʽ��
��
�����ӷ���ʽ��
��
�� Ϊ�ⶨ������е�Ԫ�ص���������������ͬ��������ηֱ���뵽50.00mL��ͬŨ�ȵ�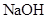 ��Һ�У���ˮԡ����������ȫ���ݳ�(���¶�����β��ֽ�)�������徭�������Ũ����������ȫ���ⶨŨ�������ӵ�������
��Һ�У���ˮԡ����������ȫ���ݳ�(���¶�����β��ֽ�)�������徭�������Ũ����������ȫ���ⶨŨ�������ӵ�������
 ���ֲⶨ�����
���ֲⶨ�����
�������Ϊ10.00g��20.00g ʱ��Ũ�������ӵ�������ͬ���������Ϊ30.00gʱ��Ũ�������ӵ�����Ϊ0.68g���������Ϊ40.00gʱ��Ũ������������䡣
 ���㣺������е�Ԫ�ص�����������
%�� ���������Ϊ15.00g�� Ũ�������ӵ�����Ϊ �� ��������������λС����
���㣺������е�Ԫ�ص�����������
%�� ���������Ϊ15.00g�� Ũ�������ӵ�����Ϊ �� ��������������λС����
1��FeS2
 ��2��
��2��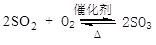
 ��3��a b d
��3��a b d
 ��4��SO3(g) + H2O(l) = H2SO4(l)����H=-130.3kJ/mol
��4��SO3(g) + H2O(l) = H2SO4(l)����H=-130.3kJ/mol
 ��5����SO2 + Br2
+ 2H2O = 4H+ + 2Br- + SO42-
��5����SO2 + Br2
+ 2H2O = 4H+ + 2Br- + SO42-
 ��14.56
2.31g
��14.56
2.31g
��������������Ҫ�������Ṥҵ���Ȼ�ѧ����ʽ����д������ȡ�
��1������ԭ���غ㼴���жϸ�����ΪFeS2��
 ��2��SO2��O2�ڽӴ��ҷ�����Ӧ����SO3��2SO2 + O2
��2��SO2��O2�ڽӴ��ҷ�����Ӧ����SO3��2SO2 + O2 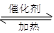 2SO3��
2SO3��
��3��ѡ��a�����������������ӷ�Ӧ�Ӵ�������ӿ췴Ӧ���ʡ�ѡ��b�����ӿ�����O2��Ũ�������SO2��ת���ʡ�ѡ��c��������ת������Ӱ�졣ѡ��d�������к���Fe2O3��������ұ������
 ��4��160g
SO3(g)��H2O(l)��Ӧ����H2SO4(l)�ų�260.6kJ��������1mol SO3(g)��H2O(l)��Ӧ����H2SO4(l)�ų�130.3kJ������������Ȼ�ѧ����ʽΪ�� SO3(g)
+ H2O(l)��H2SO4(l)����H����130.3kJ/mol��
��4��160g
SO3(g)��H2O(l)��Ӧ����H2SO4(l)�ų�260.6kJ��������1mol SO3(g)��H2O(l)��Ӧ����H2SO4(l)�ų�130.3kJ������������Ȼ�ѧ����ʽΪ�� SO3(g)
+ H2O(l)��H2SO4(l)����H����130.3kJ/mol��
��5��10gʱ˵����β���,20gʱ˵���������Ʋ���,��10.00g��20.00g ʱŨ�������ӵ�������ͬ˵���������ʽ�������İ���, �������Ƶ����ʵ���Ũ��ΪCmol/L
 ��10������Xmol�� (NH4)2SO4 ��ymol��NH4HSO4��
��10������Xmol�� (NH4)2SO4 ��ymol��NH4HSO4��
132X + 115y=10����������������������������������������.(1)
 10gʱ˵����β�����N�غ�֪
10gʱ˵����β�����N�غ�֪
n(NH3)=2X + y
 20gʱ����Ѿ����������������Ⱥ���ʽ����Ӧ����(NH4)2SO4Ϊ2Xmol��2ymol��NH4HSO4
20gʱ����Ѿ����������������Ⱥ���ʽ����Ӧ����(NH4)2SO4Ϊ2Xmol��2ymol��NH4HSO4
HSO4�� + OH�� = SO42�� + H2O
 1
1
1
1
2y 2y
 NH4+ + OH�� = NH3
+ H2O
NH4+ + OH�� = NH3
+ H2O
1 1
 50C��10��3��2y 50C��10��3��2y
50C��10��3��2y 50C��10��3��2y
���ڲ����İ���һ����
 n(NH3)=2X + y=50C��10��3��2y��������������������������.(2)
n(NH3)=2X + y=50C��10��3��2y��������������������������.(2)
30gʱ��ι�������(NH4)2SO4Ϊ3Xmol��3ymol��NH4HSO4 n(NH3)=0.68/17=0.04mol
 HSO4�� + OH�� = SO42�� + H2O
HSO4�� + OH�� = SO42�� + H2O
1 1
 3y
3y
3y
3y
NH4+ + OH�� = NH3 + H2O
 1
1
1
1
50C��10��3��3y 0.04
 ����
����
50C��10��3��3y=0.04����������������������������������������.(3)
 ����(1) (2) (3)���
����(1) (2) (3)���
X=0.02mol y=0.064mol C=4.64mol/L
 ���������ٷֺ���=
���������ٷֺ���=  ��100��=(0.04+0.064) ��14/10��100��=14.56��
��100��=(0.04+0.064) ��14/10��100��=14.56��
15gʱ����Ѿ���������(NH4)2SO4Ϊ1.5Xmol��1.5 ymol��NH4HSO4
 HSO4�� + OH�� = SO42�� + H2O
HSO4�� + OH�� = SO42�� + H2O
1 1
 1.5y
1.5y
1.5y
1.5y
NH4+ + OH�� = NH3 + H2O
 1
1
1
1
50C��10��3��1.5y 50C��10��3��1.5y
 n(NH3)= 50C��10��3��1.5y=50��4.64��10��3��1.5��0.064=0.136mol
n(NH3)= 50C��10��3��1.5y=50��4.64��10��3��1.5��0.064=0.136mol
m(NH3)=0.136��17=2.31g


 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡȪ���е»���2010�������ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�022
�Ի�����Ϊԭ����������Ĺ�������ͼ���£�
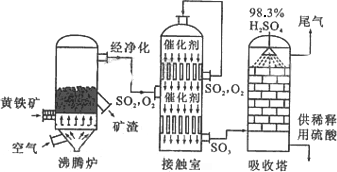
(1)��д������¯�л�����ȼ�յĻ�ѧ����ʽ��________________��
(2)�Ӵ�����2SO2(g)
��O2(g)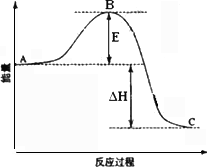
�����ݹ�������ͼ�ж�����˵����ȷ����(ѡ�������ĸ)________��
a��Ϊʹ��������ȼ�գ��轫�����
b���������������SO2��ת����
c��ʹ�ô��������SO2�ķ�Ӧ���ʺ�ת����
d������¯�ų��Ŀ����ɹ�����
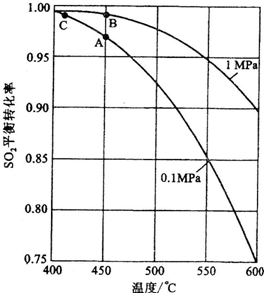
�ڷ�Ӧ�ﵽƽ��ʱ��ƽ�ⳣ������ʽK��________�������¶ȣ�Kֵ________(���������С�����䡱)��ͼ�С�H��________KJ��mol��1��
��ͼ��C���ʾ________��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ��________(��С����ޡ�)Ӱ�죮�÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B��________(����ߡ����͡�)��
�������Ӧ����v(SO2)Ϊ0.05 mol��L��1��min��1����v(O2)��________mol��L��1��min��1��
����֪�������ȼ����Ϊ��296 KJ��mol��1��������S(s)����3 mol��SO3(g)�ġ�H��________��
(3)�������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�����õ��ϸ�Ũ�ȵ�SO2����Σ�SO2�ȿ���Ϊ���������ԭ��ѭ�������ã�Ҳ�����ڹ�ҵ������������ճ�ʪ�����е�Br2��SO2����Br2�����ӷ���ʽ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��FeS2��s��+11/4O2��g��====1/2Fe2O3��s��+2SO2��g������H=��835 kJ��mol��1
��2��SO2��g��+1/2O2��g��====SO3��g������H=��98.3 kJ��mol��1
��3��SO3��g��+H2O��l��====H2SO4��l������H=��130.3 kJ��mol��1
���㽫1.0 mol FeS2�е���ȫ��ת��ΪH2SO4�������Ͽɲ�������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��ҵ���Ի�����Ϊԭ������������Ҫ��Ϊ�����ν��У����ڷ���¯�����ջ�����SO2�Ĵ���������SO3�����ա���ش����м����й����Ṥҵ�еļ������⡣
��1��������������ϵķ��������̷�Ϊԭ�ϣ������������ա���Ӧ�Ļ�ѧ����ʽΪ��2FeSO4��7H2O ![]() Fe2O3 + SO2��+ SO3��+ 14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թܡ����й��ڸ÷�Ӧ˵����ȷ����( )
Fe2O3 + SO2��+ SO3��+ 14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թܡ����й��ڸ÷�Ӧ˵����ȷ����( )

A��������Ӧ�����������ͨ��BaCl2��Һ�У������ij���ΪBaSO3��BaSO4
B��b�в�������ɫʯ����Һ���ɼ��������H+��SO42��
C��Ϊ���鷴Ӧ����һ��������Թ�c��Ӧ������Լ�ΪNaOH��Һ
D��b�����õ����������������Ϊ29.5%
(2)�ӷ���¯�г�����¯�����뾭������ϴ�ӡ���������Ӵ��ң�����ҪĿ����__________��
(3)�Ӵ������Ƚ�������ʵ�����Ƚ�����װ�á���ѧʵ����Ҳ���������Ƚ�����ʵ��ij��ʵ��Ŀ�ģ�������Һ�Ƚ���ʱͨ��ʹ�õ�������______________��
(4)�Ӵ�������Ҫ��Ӧ��SO2�Ĵ�����������������Ĺ����У�����ý(V2O5)�����ܼӿ���������������ٶȣ����˾������������⣬������Ϊ��Ӧ�����л�������һ�������м���(��ͼ)��c���Ļ�ѧ����ʽ�ɱ�ʾΪ_______________________��

(5) ��ҵ����������Ϊԭ����������������β�����˺���N2��O2�⣬������SO2������SO3��������Ϊ�˱���������ͬʱ������Ṥҵ���ۺϾ���Ч�棬Ӧ�����ܽ�β���е�SO2ת��Ϊ���õĸ���Ʒ����β��ͨ���ĩ״��̼��ƻ���ʯ�ҵ�����Һ�У�����һϵ�д�����õ�һ����Է�������Ϊ172�Ļ���ԭ��J����д��J�Ļ�ѧʽ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com