
| ���� | �Ҷ��� | �Ҷ��ᾧ�� |
| ����ʽ | H2C2O4 | H2C2O4��2H2O |
| ��ɫ״̬ | ��ɫ���� | ��ɫ���� |
| �ܽ�ȣ�g�� | 8.6��20�棩 | �� |
| �۵㣨�棩 | 189.5 | 101.5 |
| �ܶȣ�g��cm��3�� | 1.900 | 1.650 |


 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д� һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
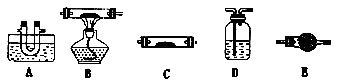
| A����������й©ʱ��Ѹ���뿪�ֳ������������ʹ�ȥ |
| B����Ϊ�����Ȼ����ܽ�ʱ��ЧӦ�������Կ���������ƿ���ܽ��Ȼ��ƹ��� |
| C���ü��ȵķ��������Ȼ��ƺ��Ȼ�粒��� |
| D�����ܽ⡢���˵ķ�����ȥ�����е��Ȼ��ƺ��Ȼ�þ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��Һ��
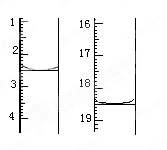
��Һ�� δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ���
δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ��� )��
)��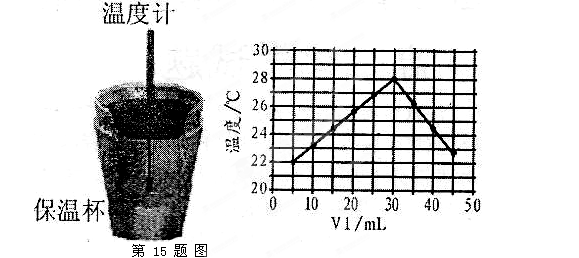 (1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��
(1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��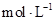
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ�鲽��(��Ҫ��д�����������) | Ԥ������ͽ��� |
| �ٲⶨ����ʵ���õ�Fe��NO3��3��Һ��pHֵ | |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 2CrO42��+2H+���ƣ����µζ��յ��ͺ�
2CrO42��+2H+���ƣ����µζ��յ��ͺ�| ʵ����� | FeSO4��Һ���������/mL | |
| �ζ�ǰ | �ζ��� | |
| 1 | 0.10 | 16.20 |
| 2 | 0.30 | 15.31 |
| 3 | 0.20 | 15.19 |
�鿴�𰸺ͽ���>>
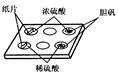
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
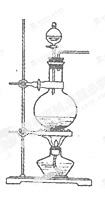
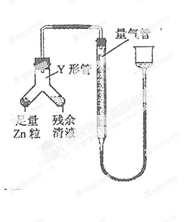
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| װ�� | 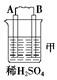 | 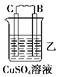 | 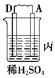 |
| ���� | ���۽���A�����ܽ� | C���������� | A����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com