
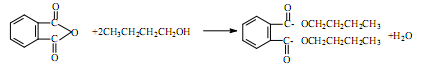
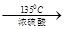 = CH3(CH2)3O(CH2)3CH3+H2O
= CH3(CH2)3O(CH2)3CH3+H2O 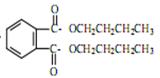 +2NaOH
+2NaOH 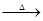 2CH3(CH2)2CH2OH+2H2O+
2CH3(CH2)2CH2OH+2H2O+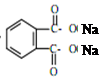
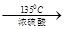 = CH3(CH2)3O(CH2)3CH3+H2O��DBP������������Һ��ˮ��ķ���ʽΪ
= CH3(CH2)3O(CH2)3CH3+H2O��DBP������������Һ��ˮ��ķ���ʽΪ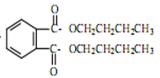 +2NaOH
+2NaOH 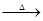 2CH3(CH2)2CH2OH+2H2O+
2CH3(CH2)2CH2OH+2H2O+ ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��������������մ����������ǽ������� |
| B��1����=10��10�� |
| C��ﯺϽ��Ӳ�ȱȴ��Ҫ�� |
| D���ձ������˵�վ�ı�ը��������ﯺϽ��ڸ�������ˮ������Ӧ������������ը���� |
�鿴�𰸺ͽ���>>
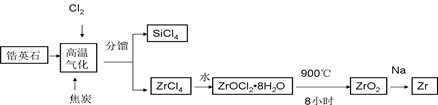
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
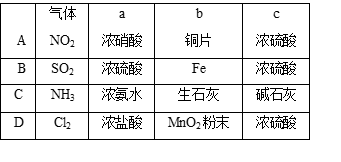
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ���� | �״� | ������ | ��������� |
| �е㣯�� | 64.7 | 249 | 199.6 |
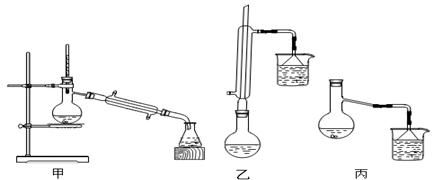
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
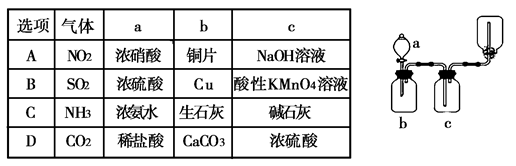
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
|
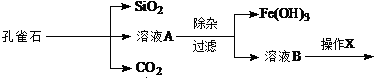
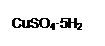 Ϊ����й����⣬��ȤС��ͬѧ����й����ʳ�����pH�������£�
Ϊ����й����⣬��ȤС��ͬѧ����й����ʳ�����pH�������£�| ���� | pH (��ʼ����) | pH(��ȫ����) |
| Fe(OH)3 | 1.9 | 3.2 |
| Fe(OH)2 | 7.0 | 9.0 |
| Cu(OH)2 | 4.7 | 6.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Fe(OH)3�� | B��FeCl2 | C��Fe(OH)2 | D��FeCl3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
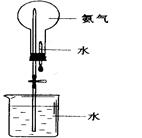
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com