��2008?�㶫��ͭ����Ȼ������ڶ��ֿ�ʯ�У��磺
| ��ʯ���� |
��ͭ�� |
��ͭ�� |
��ͭ�� |
��ȸʯ |
| ��Ҫ�ɷ� |
CuFeS2 |
Cu5FeS4 |
Cu2S |
CuCO3?Cu��OH��2 |
���ϱ�����ͭ�������У�ͭ�������ٷֺ�����ߵ���
Cu2S
Cu2S
��
�ڹ�ҵ���Ի�ͭ��Ϊԭ�ϣ����û�������������ͭ���ù��յ��м���̻ᷢ����Ӧ��2Cu
2O+Cu
2S
6Cu+SO
2������Ӧ����������
Cu2O��Cu2S
Cu2O��Cu2S
��
��SO
2β��ֱ���ŷŵ���������ɻ�����Ⱦ�ĺ����
�����������ж����壬������������ˮ���γ�������Һ������ˮ���£��Ϳ����γ�����
�����������ж����壬������������ˮ���γ�������Һ������ˮ���£��Ϳ����γ�����
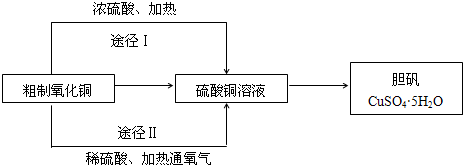
��������β���ɵõ��м�ֵ�Ļ�ѧƷ��д������1�����1���ε�����
���ᣬ����泥�
���ᣬ����泥�
��
�ܻ�ͭ��������õ��Ĵ�ͭ������Fe��Ag��Au�Ƚ������ʣ����һ�����õ�ⷨ���ƣ��������ͭ���õ���ͭ��ԭ����
��ͭ���õ���ͭ��ԭ���������У���ͭ����������ͭ�������������Ϊ����ͭ��Һ�������Ϸ���������Ӧ��Cuʧȥ���ӣ�ʹCu���ʱ�ΪCu 2+������Һ��Cu-e-�TCu 2+�������Ϸ�����ԭ��Ӧ��Cu 2+�õ�����������������Cu���ʣ�Cu 2++e-�TCu���Ӷ��ﵽ����Cu��Ŀ�ģ�
��ͭ���õ���ͭ��ԭ���������У���ͭ����������ͭ�������������Ϊ����ͭ��Һ�������Ϸ���������Ӧ��Cuʧȥ���ӣ�ʹCu���ʱ�ΪCu 2+������Һ��Cu-e-�TCu 2+�������Ϸ�����ԭ��Ӧ��Cu 2+�õ�����������������Cu���ʣ�Cu 2++e-�TCu���Ӷ��ﵽ����Cu��Ŀ�ģ�
��
���±��У��Գ��������ȷ�Լ������������ϵ���ж϶���ȷ����
AD
AD
������ĸ����
| ѡ�� |
������ |
������ |
�ж� |
| A |
ͭ�̵����ɷ��Ǽ���ͭ |
����ϡ�����ͭ�������ͭ�� |
��ԣ���ԣ��� |
| B |
ͭ�����γ����ܵ�����Ĥ |
ͭ��������ʢ��Ũ���� |
��ԣ���ԣ��� |
| C |
����ͭ���� |
����ͭ���ϵ������ڳ�ʪ�����в������� |
��ԣ���ԣ��� |
| D |
��ɫ����ͭ��������ת��Ϊ��ɫ����ͭ��ĩ�������仯 |
����ͭ��Һ��������Ӿ�ص������� |
�������ԣ��� |