
ʵ������ȼ�շ��ⶨij�ְ�����(CxHyOzNp)�ķ�����ɣ�ȡm g���ְ�������ڴ����г��ȼ�գ�����CO2��H2O��N2.�ְ���ͼװ�ý���ʵ�飬������������⣺
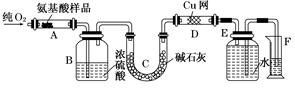
(1)ʵ�鿪ʼʱ������Ҫͨ��һ��ʱ�����������������_____________________��
(2)����װ������Ҫ���ȵ�������________(����ĸ)������ʱӦ�ȵ�ȼ________���ľƾ��ƣ�
(3)Aװ���з�����Ӧ�Ļ�ѧ����ʽΪ_____________________________��
(4)װ��D��������____________________________��
(5)��ȡN2�����ʱ��Ӧע���__________________����________________________.
(6)ʵ���в��N2�����ΪV mL(������ɱ�״��)��Ϊȷ���˰�����ķ���ʽ������Ҫ���й�������____________��
A������CO2����������� B������ˮ������
C��ͨ��O2����� D�����������Է�������
(1)�ų���ϵ�еĿ���(��Ҫ��N2)��(2)A��D��D
(3)
(4)����δ��Ӧ��O2����֤�����ռ�����������N2
(5)�ٵ���ʹE��FҺ����ƽ��������Ӧ�밼Һ����͵����С�(6)ABD
��������
�����������ʵ����ŨH2SO4����������ˮ��ʢŨH2SO4��ƿ���ص�������Ϊˮ����������ʯ�����ص�������ΪCO2��������ͨ��ͭ����ȫ����O2��ͨ����ˮ����N2�������������������������Ʒ��ʵ��ʽ����֪�����������Է��������������������ʽ��Ϊ��֤ʵ��ⶨ��ȷ�ԣ����ȡһϵ�д�ʩ��ʵ��ǰ��ͨ�������ž�����������Ӱ��N2����IJⶨ��
���㣺������ȼ�շ�ȷ���л��������ɵ�ʵ��̽��
�����������Ǹ߿��еij���ͼ�����Ҫ�Ŀ���֮һ�������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ��������������ʵ��������ָ���������ڵ���ѧ����ѧϰ��Ȥ������ѧ����ѧ�����������������ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ��еĻ��ṩһЩ�µ���Ϣ�����Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ�


 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д� �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

������������⣺
(1)ʵ�鿪ʼʱ������Ҫͨ��һ��ʱ�����������������______________________��
(2)����װ������Ҫ���ȵ�������______________________(����ĸ��գ���ͬ)������ʱӦ�ȵ�ȼ__________���ľƾ��ơ�
(3)Aװ���з�����Ӧ�Ļ�ѧ����ʽ��____________________________��
(4)װ��D��������_____________________________��
(5)��ȡN2�����ʱ��Ӧע���______________________����______________________��
(6)ʵ���в��N2�����ΪV mL(��״��)��Ϊȷ���˰�����Ļ�ѧʽ������Ҫ���й�������_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��������ѧ��һDZ�ܰ�����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(14��)ʵ������ȼ�շ��ⶨij�����л���A�ķ�����ɣ��ⶨװ����ͼ(����̨�����С��ƾ��Ƶ�δ����)��
ȡ17.1 g A����װ���У�ͨ�����O2ȼ�գ�����CO2��H2O����ش������й����⣺
(1)ͨ�����O2��Ŀ����______________________________________________��
(2)Cװ�õ�������__________________________________________��
Dװ�õ�������_____________________________________________��
(3)ͨ����ʵ�飬�ܷ�ȷ��A���Ƿ�����ԭ�ӣ�________��
(4)��A��Ħ������Ϊ342 g/mol��Cװ������9.99 g��Dװ������26.4 g����A����ʽΪ____________��
(5)д��Aȼ�յĻ�ѧ����ʽ_____________________________________��
(6)A�ɷ���ˮ�ⷴӦ��1 mol A��ˮ������2 molͬ���칹�壬��A�ڴ���������ˮ��Ļ�ѧ����ʽΪ_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��������ѧ��һDZ�ܰ�����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(14��)ʵ������ȼ�շ��ⶨij�����л���A�ķ�����ɣ��ⶨװ����ͼ(����̨�����С��ƾ��Ƶ�δ����)��

ȡ17.1 g A����װ���У�ͨ�����O2ȼ�գ�����CO2��H2O����ش������й����⣺
(1)ͨ�����O2��Ŀ����______________________________________________��
(2)Cװ�õ�������__________________________________________��
Dװ�õ�������_____________________________________________��
(3)ͨ����ʵ�飬�ܷ�ȷ��A���Ƿ�����ԭ�ӣ�________��
(4)��A��Ħ������Ϊ342 g/mol��Cװ������9.99 g��Dװ������26.4 g����A����ʽΪ____________��
(5)д��Aȼ�յĻ�ѧ����ʽ_____________________________________��
(6)A�ɷ���ˮ�ⷴӦ��1 mol A��ˮ������2 molͬ���칹�壬��A�ڴ���������ˮ��Ļ�ѧ����ʽΪ_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������ȼ�շ��ⶨij������CxHyOzNp�ķ�����ɡ�ȡmg���ְ�������ڴ����г��ȼ�գ�����CO2��H2O��N2��ʵ��װ������ͼ��

��ش������й����⣺
��1��ʵ�鿪ʼʱ������Ҫͨ��һ��ʱ����������������ǣ�
��
��2������װ������Ҫ���ȵ������� ������ĸ����ͬ������ʱӦ�ȵ�ȼ
���ľƾ��ơ�
��3��Aװ���з�����Ӧ�Ļ�ѧ����ʽ�ǣ�
��4��װ��D�������ǣ�
��5����ȡN2���ʱ��Ӧע�⣺�� ��
�� ��
��6��ʵ���в��N2�����VmL��������Ϊ��״������Ϊȷ���˰�����ķ���ʽ������Ҫ���й������� ������ĸ��գ���
A�����ɶ�����̼���������
B�����������Է�������
C��ͨ�����������
D������ˮ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com