
�е��װ����ͼ��ͼ��Bװ��ʢ1 L��2 mol/L��Na2SO4��Һ��Aװ����ʢ1 L��2 mol/L��AgNO3��Һ��ͨ�����ʪ�ĵ��۵�KI��ֽ��C�˱���ɫ�����һ��ʱ����Իش�
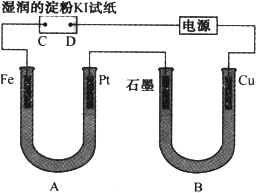
(1)A�������ĵ缫��ӦʽΪ________��������ӦʽΪ________��
(2)��B�й۲쵽��������________��
(3)�����£����ӵ�ʼ��ʱ��Ϊtʱ��A��Bװ���й��ռ�������0.168 L(��״��)����������������������Ӧ����������Һ����仯���Բ��ƣ�����tʱ��A��Һ��pHΪ________��
|
���� ����˼·�뼼�ɣ������е��۱�����һ�����ۣ��Դ�Ϊ����չ����ע����������Դ���������ĵ缫Ϊ�������ڴ����У���Ȼ�ǰ��յ����ķ������Ǵ����������������жϵ����еĵ缫���������������� ����(1)ʪ��ĵ��۵�KI��ֽ��C�˱���ɫ����C�˲�����Cl2����Cl���������ŵ磬C����������Pt��������Cu��������Fe��������ʯī��������A��������Ag+�ŵ磬����Ag������OH���ŵ�ų�O2�� ����(2)B������Cu�ŵ��ΪCu2+����Һ��ɫ����������Һ����ΪNa+��࣬H+������Cu2+�淴Ӧ���ж����ɣ������������Ϸŵ��Ӧ��H+��ΪH2�� ���� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ���ݰ���2007�������ѧ�������¿��Ծ� ���ͣ�022
| |||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��1��A�з�����Ӧ�Ļ�ѧ����ʽΪ_______________________________________________��
��2����B�й۲쵽������Ϊ_______________________________________________________
____________________________________________________________________��
��3�������£����ӵ�ʼ��ʱ��Ϊtʱ��A��Bװ���й��ռ�������0.168 L����״��������������������������Ӧ����������Һ����仯���Բ��ƣ�����tʱ��A��Һ��pHΪ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��1����Դ��xΪ_________����
��2��B���������ĵ缫��Ӧʽ��_____________��
��3����״��ʱ�������A�й��ռ���0.56 L���壬��A���е�pHΪ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
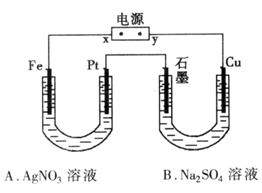
�ٵ�Դx��Ϊ____________��
��B���������缫��Ӧ����_______________________________��
�۱��ʱ�����A�й��ռ���0.56L���壬��A���е�pHΪ_______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com