
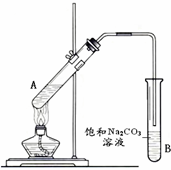
 �����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
�����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺���� ��1�������ӷ����Ҵ����ܶȱ�Ũ�����С�����Ũ�����ϡ�ͽ��н��
��2��Ũ�������˴����ú���ˮ���ã�
��3������̼������Һ�����ᷴӦ��ȥ���ᡢͬʱ���������������ܽ�ȣ����ڷֲ㣻
��4�����ݵ�������Һ���¿��ܷ�������������
��5�����뻥�����ܵ�Һ�壬���÷�Һ�ķ������룻
��6��Һ�����Ҫ�����Ƭ����ֹ���У�
��� �⣺��1��Ũ�����ϡ���ǽ�Ũ�������ˮ�У��ӱ߽��裬Ǩ�Ƶ��˴��������ڴ��Թ��м����Ҵ���Ȼ������������ע�����ᣬ�����Ͻ��裬�����װ���Ҵ���Ũ����Ļ����Ĵ��Թ��м������ᣬ
�ʴ�Ϊ���Ҵ���Ũ������
��2���������Ҵ�����������Ӧ����Ũ�������������÷�ӦΪ���淴Ӧ��Ũ������ˮ����ƽ���������������������ƶ���Ũ���������Ϊ��������ˮ����
�ʴ�Ϊ����������ˮ����
��3���Ʊ���������ʱ���ñ���̼������Һ��Ŀ�����кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У�������������������ζ���ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
�ʴ�Ϊ���кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У�������������������ζ���ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
��4�����ܲ��ܲ�����Һ�У�����Ҫ���ڱ���̼������Һ��Һ���ϣ�����Һ���¿��ܷ���������
�ʴ�Ϊ��������
��5��������������ʱ�Ƚ�ʢ�л������Թܳ�����ñ���̼������Һ�кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У��ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����÷ֲ��ȡ�ϲ������������
�ʴ�Ϊ����Һ��
��6��Һ�����Ҫ�����Ƭ�������������ģ��ɷ�ֹ���Թ���Һ�屩�ж�������ܣ�
�ʴ�Ϊ����ֹ���Թ���Һ�屩�ж�������ܣ�
���� ���⿼�������������Ʊ�����Ŀ�Ѷ��еȣ��漰�������ϴ�ע��ʵ����Һ�����ơ�����̼������Һ�������Լ�������Ӧ�Ļ���������������ѧ���������������������Ӧ����ѧ֪ʶ���ʵ�������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
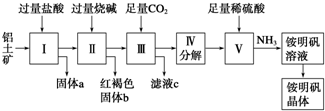
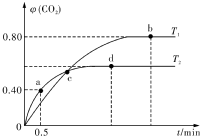 ����I2O5����CO��Ⱦ�ķ�ӦΪ��5CO��g��+I2O5��s���T5CO2��g��+I2��s������ͬ�¶��£���װ������I2O5�����2L�����ܱ�������ͨ��4molCO�����CO2�����������ʱ��t�仯������ͼ����ش�
����I2O5����CO��Ⱦ�ķ�ӦΪ��5CO��g��+I2O5��s���T5CO2��g��+I2��s������ͬ�¶��£���װ������I2O5�����2L�����ܱ�������ͨ��4molCO�����CO2�����������ʱ��t�仯������ͼ����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
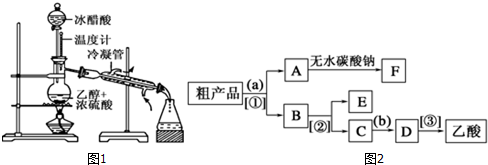
 ������Ҵ���Ӧ��װ����ͼ��ʾ�����Թ������3mL�Ҵ���Ȼ��һ��ҡ����һ�������ؼ���2mLŨ�����2mL�����ᣬ�þƾ���С�ľ��ȵؼ���10min����������������������ͨ�뵽����̼������Һ��Һ���ϣ�
������Ҵ���Ӧ��װ����ͼ��ʾ�����Թ������3mL�Ҵ���Ȼ��һ��ҡ����һ�������ؼ���2mLŨ�����2mL�����ᣬ�þƾ���С�ľ��ȵؼ���10min����������������������ͨ�뵽����̼������Һ��Һ���ϣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 ������������һ����ɫ��Һ�塢�����Ҵ������ѻ��ܣ�������ˮ������������ˮ�㾫�����쾫�ͣ����Ʊ�ԭ�����£�
������������һ����ɫ��Һ�塢�����Ҵ������ѻ��ܣ�������ˮ������������ˮ�㾫�����쾫�ͣ����Ʊ�ԭ�����£�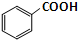 +C2H5OH$\stackrel{H_{2}SO_{4}}{?}$
+C2H5OH$\stackrel{H_{2}SO_{4}}{?}$ +H2O
+H2O| ���� | ��ɫ״̬ | �ܶ�/g•cm-3 | �۵�/�� | �е�/�� |
| ������* | ��ɫ���� | 1.2659 | 122 | 249 |
| ���������� | ��ɫҺ�� | 1.05 | -34.6 | 212.6 |
| ���� | ��ɫҺ�� | 1.0492 | 16.6 | 117.9 |
| �Ҵ� | ��ɫҺ�� | 0.789 | -117.3 | 78.5 |
| �������� | ��ɫҺ�� | 0.894-0.898 | -83.6 | 77.1 |
| ���� | ��ɫҺ�� | 0.713 | -116.3 | 34.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
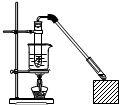
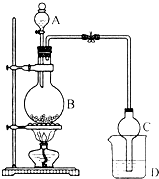 ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ����֪��
ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ����֪��| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е㣨�棩 | 34.7 | 78.5 | 118 | 77.1 |
 CH3CO18OCH2CH3+H2O��
CH3CO18OCH2CH3+H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
�� ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com