
������������������ 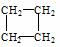 ��
�� 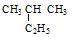 ������ ������ ��
������ ������ ��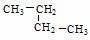 �����л�Ϊͬ���칹����� ������ţ��������֮��Ĺ�ϵΪ ��
�����л�Ϊͬ���칹����� ������ţ��������֮��Ĺ�ϵΪ ��
�� �� �� ���������ʰ����ǵķе��ɸߵ��͵�˳��������ȷ���� ������ţ�
��ij��A���л���ѧ��ҵ�Ļ���ԭ�ϣ������������������һ�����ҵ�ʯ�ͻ�����չˮƽ��A��һ�������¿ɷ�����ͼ��ʾ��ת������ش��������⣺
��1��д��A�ĵ���ʽ ��E�Ľṹ��ʽΪ
��2��д�����з�Ӧ��ѧ����ʽ����ע���ۢݷ�Ӧ����
�� _____________________________________________
�� _____________________ ����Ӧ����________��
�� _____________________ ����Ӧ����________��
��3����ȥB�л��е���������A�����õ��Լ�Ϊ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڸ��¡���ѹ�ʹ��������µ��ܱ������У����з�Ӧ��N2(g)+3H2(g) 2NH3(g) (����Ӧ����)�������й�˵������ȷ����( )
2NH3(g) (����Ӧ����)�������й�˵������ȷ����( )
A��ʹ�ô�����Ϊ������ѧ��Ӧ����
B������������Ũ�ȿ�����Ӧ����
C�������������£�������ȫ��ת��Ϊ����
D���ﵽƽ��ʱ����ϵ�и����ʵ�Ũ�Ȳ��ٸı�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ�����ڱ���Ԫ����������ѧϰ���о�������ʵ�����к���Ҫ�����á��±��г��ˢ١������Ԫ�������ڱ��е�λ�á�
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� |
��ش�
(1)�����Ԫ�طֱ���(��Ԫ�ط���)�� ���� ���� ���� ���� ���� ���� ���� ���� �����л�ѧ��������õ��� ��
(2)�ڢ١��ڡ�������Ԫ�ص��������Ӧ��ˮ�����У�������ǿ���� (�ѧʽ)��
(3)�١��ڡ�������Ԫ�ذ�ԭ�Ӱ뾶�ɴ�С��˳������Ϊ (��Ԫ�ط���)��
(4)��Ԫ�ص��⻯���� (�ѧʽ)�����⻯���ڳ�������ڷ�����Ӧ�����ӷ���ʽΪ ��������Һ��pH (�����������������)7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ������о���һ�ֻ�������ȫ�Ĵ��ⷽ������ԭ���ɱ�ʾΪ��
NaHCO3+H2  HCOONa+H2O�����й�˵����ȷ����
HCOONa+H2O�����й�˵����ȷ����
A�����⡢������̾��������仯 B����������У�NaHCO3������
C��NaHCO3�������Ӽ����ۼ� D����������У�ÿ����0.1molH2O�ų�2.24L��H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���¶Ⱥ�һ��������ܱ������з������·�Ӧ��A2(s)+ B2(g) 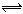 2AB(g) ����Ӧ�ﵽƽ��״̬�ı�־��
2AB(g) ����Ӧ�ﵽƽ��״̬�ı�־��
A��V����B2��==2V����AB�� B����λʱ��������2molAB��ͬʱ����1mol��B2
C�������ڵ���ѹǿ����ʱ����仯 D�������ܶȲ�����ʱ��仯���仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1���ϳɰ���ҵ������������Ȼ����ˮ��Ӧ�Ʊ�������Ҫ��ӦΪ��
CH4(g)+ 2H2O(g)  CO2(g)+4H2(g)
CO2(g)+4H2(g)
��Ӧ�����������仯��ͼ��ʾ��
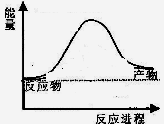 ��÷�ӦΪ ��Ӧ������ȡ����ȡ���
��÷�ӦΪ ��Ӧ������ȡ����ȡ���
����֪���ƻ�1mol��ѧ����Ҫ���յ��������±���ʾ��
| ��ѧ�� | C��H | O��H | C=O | H��H |
| ����������kJ/mol�� | a | b | c | d |
��÷�Ӧ���ջ�ų�����Ϊ kJ(�ú�a b c d��ĸ�Ĵ���ʽ��ʾ)
��2��ij�¶��£�10L�ܱ������г���2mol CH4��3mol H2O(g)������
CH4(g)+ 2H2O(g)  CO2(g)+4H2 (g)��Ӧ�� ��Ӧ���е�4sʱCO2��Ũ��Ϊ0.08mol/L���ٹ�һ��ʱ�䷴Ӧ��ƽ�⣬ƽ��ʱ������ѹǿ����ʼʱ��1.4����
CO2(g)+4H2 (g)��Ӧ�� ��Ӧ���е�4sʱCO2��Ũ��Ϊ0.08mol/L���ٹ�һ��ʱ�䷴Ӧ��ƽ�⣬ƽ��ʱ������ѹǿ����ʼʱ��1.4����
�� �� ǰ4s��H2O(g)Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ���٣��� 4sʱ�����������H2���������Ϊ���٣��� ƽ��ʱ��CH4��Ũ���Ƕ��٣�
��Ҫ��д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪A ��һ�ַ�����Ϊ28����̬��������AΪ��Ҫԭ�Ϻϳ�һ�־��й���ζ������E����ϳ�·������ͼ��ʾ��
 |
��ش��������⣺
��1��д��A�Ľṹ��ʽ ��
��2��B��D�����еĹ��������Ʒֱ��� �� ��
��3������B���Ա�ֱ������ΪD����Ҫ������Լ��� ��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
�� ����Ӧ���ͣ� ��
�� ����Ӧ���ͣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��̼����(2Na2CO3��3H2O2)��һ�ּ�ϴ�ӡ�Ư�ס�ɱ����һ�����ϵƯ�����þ������Na2CO3��H2O2��˫�����ʡ�����ͼ2װ���Ʊ���̼���ƣ�����ˮԡ�г�ַ�Ӧ��ͼ1���̿ɻ�ù�̼���Ʋ�Ʒ��
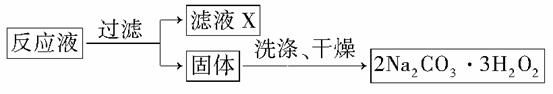
ͼ1

(1)��ѹ��Һ©����֧�ܵ�������________________________��
(2)�Ʊ���̼���ƵĹؼ���______________________________��
(3)������ƹ�̼���Ƶ�ˮ�к��������ӣ�����������ϴ�Ӽ���ȥ��������������ȫʧȥɱ�����á��Է������е�ԭ��_____________
(д������һ�ּ��ɣ��÷���ʽ��ʾ)��
(4)ij��ѧѧϰС��Ϊ�˶���̽�������Ӷ���������Ư���IJ���Ӱ�죬ȡ��Ư��100 mL������25 g FeCl3���壬����������ɫ��ζ���壬������ƿ�ռ����塣��ѡ�������Լ���ʵ����Ʒ�������ɷֵ�̽�����̣�0.1 mol/L NaOH��Һ��8.0 mol/L NaOH��Һ������ʯ��ˮ��0.01 mol/L KMnO4��Һ��BaCl2ϡ��Һ��Ʒ����Һ������ˮ��ľ�����ƾ��ơ����ϴ��ƿ��
��������裺�Ը�����ɷ�����������衣
����1��������O2��
����2��������________________��
����3��������CO2��
����Ʒ��������ʵ�鷽��֤����ļ��裬���±������ʵ�鲽�衢Ԥ����������ۡ�
| ʵ�鲽�� | Ԥ����������� |
| ����������ͨ��ʢ��____________��ϴ��ƿ�У�____________ | ��____________�� ��____________�� ��____________ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com