
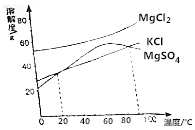
 ĪŅ¹śŹ³ŃĪÓŠ80%Ą“×Ōµ×ĻĀ¾®ŃĪŗĶŃŅŃĪ£¬ÓĆŃĪ¾®Ė®É¹ŃĪŹĒÖĘČ”Ź³ŃĪµÄ³£ÓĆ·½·Ø£®
ĪŅ¹śŹ³ŃĪÓŠ80%Ą“×Ōµ×ĻĀ¾®ŃĪŗĶŃŅŃĪ£¬ÓĆŃĪ¾®Ė®É¹ŃĪŹĒÖĘČ”Ź³ŃĪµÄ³£ÓĆ·½·Ø£®·ÖĪö £Ø1£©·ÖĄė¹ĢŅŗ»ģŗĻĪļÓĆ¹żĀĖ£»“ÓČÜŅŗÖŠĢįČ”ČÜÖŹÓĆÕō·¢½į¾§µÄ²Ł×÷£»
£Ø2£©¢Łøł¾ŻĶ¼ÖŠ90”ęŅŌMgSO4µÄČܽā¶Č±ä»Æ·ÖĪö£»
¢ŚĪö³öKCl¾§ĢåŹ±£¬MgCl2ŗĶMgSO4µÄČܽā¶ČŅŖ“óÓŚKCl£»
£Ø3£©¼ģŃéĆ¾Ąė×ÓÓĆĒāŃõ»ÆÄĘ£¬»įÉś³É°×É«³Įµķ£®
½ā“š ½ā£ŗ£Ø1£©³żČ„ŃĪĖ®ÖŠÄąĶĮ£¬ŹĒ·ÖĄė¹ĢŅŗ»ģŗĻĪļ£¬Ęä²Ł×÷ĪŖ¹żĀĖ£»ŃĪĖ®É¹ŃĪ£¬“ÓČÜŅŗÖŠĢįČ”æÉČÜŠŌµÄČÜÖŹ£¬Ęä²Ł×÷ĪŖÕō·¢½į¾§£»
¹Ź“š°øĪŖ£ŗ¹żĀĖ£»Õō·¢½į¾§£»
£Ø2£©¢ŁÓÉĶ¼ÖŠ90”ęŅŌMgSO4µÄČܽā¶Č±ä»ÆæÉÖŖ£¬¼ÓČȵ½90”ęŅŌÉĻ£¬MgSO4µÄČܽā¶ČĖę×ÅĪĀ¶ČÉżø߶ų¼õŠ”£¬¶ųKCl”¢MgCl2µÄČܽā¶ČŌö“ó£¬ĖłŅŌ½«Ā±Ė®¼ÓČȵ½90”ęŅŌÉĻÕō·¢ŹŹĮæĖ®·Ö£¬×īĻČŠĪ³É±„ŗĶČÜŅŗµÄŹĒMgSO4£¬¼“MgSO4×īĻČĪö³ö£»
¹Ź“š°øĪŖ£ŗMgSO4£»¼ÓČȵ½90”ęŅŌÉĻ£¬MgSO4µÄČܽā¶ČĖę×ÅĪĀ¶ČÉżø߶ų¼õŠ”£»
¢ŚĪö³öKCl¾§ĢåŹ±£¬MgCl2ŗĶMgSO4µÄČܽā¶ČŅŖ“óÓŚKCl£¬ÓÉĶ¼ĻóæÉÖŖ£¬ŌŚ20”«90”ꏱ£¬KClµÄČܽā¶ČŠ”ÓŚĮķĶāĮ½ÖÖĪļÖŹµÄČܽā¶Č£¬ĖłŅŌĪö³öKCl¾§ĢåµÄĪĀ¶Č·¶Ī§ŹĒ20”«90”ę£»
¹Ź“š°øĪŖ£ŗ20”«90£»
£Ø3£©ĪŖ¼ģŃéÖʵƏ³ŃĪÖŠŹĒ·ńŗ¬ÓŠMg2+£¬æɽ«ÉŁĮ澧ĢåÅä³ÉČÜŅŗ£¬Č»ŗó¼ÓČČĒāŃõ»ÆÄĘČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³ÉĖµĆ÷ŗ¬ÓŠĆ¾Ąė×Ó£¬Ęä·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ2OH-+Mg2+=Mg£ØOH£©2”ż£»
¹Ź“š°øĪŖ£ŗNaOH£»2OH-+Mg2+=Mg£ØOH£©2”ż£®
µćĘĄ ±¾Ģāæ¼²éĮĖĪļÖŹ·ÖĄėĢį“æµÄŹµŃé·½°øÉč¼Ę”¢Ąė×Ó·½³ĢŹ½µÄŹéŠ“”¢Čܽā¶ČÓėĪĀ¶ČµÄ¹ŲĻµĶ¼Ļó£¬ĢāÄæ×ŪŗĻŠŌ½ĻĒ棬ÄѶČÖŠµČ£¬²ąÖŲÓŚæ¼²éѧɜµÄ·ÖĪöÄÜĮ¦ŗĶŹµŃéĢ½¾æÄÜĮ¦£¬×¢Ņā°ŃĪÕĢāÖŠĒśĻßÉĻĪļÖŹµÄČܽā¶ČÓėĪĀ¶ČµÄ¹ŲĻµ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Į½ČÜŅŗµÄµ¼µēÄÜĮ¦ŗĶpHÖµ¾łĻąĶ¬ | |
| B£® | ÖŠŗĶĮ½ČÜŅŗ£¬ĻūŗÄNaOHµÄĪļÖŹµÄĮæĻąĶ¬ | |
| C£® | ·Ö±šÓė×ćĮæµÄZnĶźČ«·“Ó¦£¬ŃĪĖį²śÉśµÄĖŁĀŹæģ£¬ĒāĘų¶ą | |
| D£® | µ±°ŃĮ½ÖÖĖįø÷10mL»ģŗĻŗ󣬼Ó10mL 0.1mol•L-1µÄNaOH£¬ŌņÓŠc£ØH+£©=c£ØCH3COO-£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
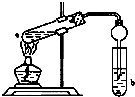 ČēĶ¼£¬ŌŚŹŌ¹ÜaÖŠĻČ¼ÓČė3mLµÄŅŅ“¼£¬±ßŅ”±ß»ŗĀż¼ÓČė2mLÅØĮņĖį£¬ŌŁ¼ÓČė2mLĪŽĖ®ŅŅĖį£¬ÓĆ²£²£°ō³ä·Ö½Į°čŗó½«ŹŌ¹Ü¹Ģ¶ØŌŚĢś¼ÜĢØÉĻ£¬ŌŚŹŌ¹ÜbÖŠ¼ÓČėŹŹĮ汄ŗĶĢ¼ĖįÄĘČÜŅŗ£®Į¬½ÓŗĆ×°ÖĆ£¬ÓĆ¾Ę¾«µĘ¶ŌŹŌ¹Ü¼ÓČČ£¬µ±¹Ū²ģµ½ŹŌ¹ÜbÖŠÓŠĆ÷ĻŌĻÖĻóŹ±Ķ£Ö¹ŹµŃ飮
ČēĶ¼£¬ŌŚŹŌ¹ÜaÖŠĻČ¼ÓČė3mLµÄŅŅ“¼£¬±ßŅ”±ß»ŗĀż¼ÓČė2mLÅØĮņĖį£¬ŌŁ¼ÓČė2mLĪŽĖ®ŅŅĖį£¬ÓĆ²£²£°ō³ä·Ö½Į°čŗó½«ŹŌ¹Ü¹Ģ¶ØŌŚĢś¼ÜĢØÉĻ£¬ŌŚŹŌ¹ÜbÖŠ¼ÓČėŹŹĮ汄ŗĶĢ¼ĖįÄĘČÜŅŗ£®Į¬½ÓŗĆ×°ÖĆ£¬ÓĆ¾Ę¾«µĘ¶ŌŹŌ¹Ü¼ÓČČ£¬µ±¹Ū²ģµ½ŹŌ¹ÜbÖŠÓŠĆ÷ĻŌĻÖĻóŹ±Ķ£Ö¹ŹµŃ飮²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
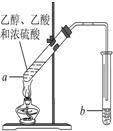 ŗģĘĻĢŃ¾ĘĆÜ·ā“¢“ꏱ¼äŌ½³¤£¬ÖŹĮæŌ½ŗĆ£¬ŌŅņÖ®Ņ»ŹĒ“¢“ę¹ż³ĢÖŠÉś³ÉĮĖÓŠĻćĪ¶µÄõ„£®ŌŚŹµŃéŹŅŅ²æÉŅŌÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆÖĘČ”ŅŅĖįŅŅõ„£¬Ēė»Ų“šĻĀĮŠĪŹĢā£®
ŗģĘĻĢŃ¾ĘĆÜ·ā“¢“ꏱ¼äŌ½³¤£¬ÖŹĮæŌ½ŗĆ£¬ŌŅņÖ®Ņ»ŹĒ“¢“ę¹ż³ĢÖŠÉś³ÉĮĖÓŠĻćĪ¶µÄõ„£®ŌŚŹµŃéŹŅŅ²æÉŅŌÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆÖĘČ”ŅŅĖįŅŅõ„£¬Ēė»Ų“šĻĀĮŠĪŹĢā£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CH4ŗĶC2H4 | B£® | C2H2ŗĶC2H4 | C£® | C2H4ŗĶC2H6 | D£® | C4H8ŗĶC3H6 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 25”ꏱ£¬1LpH=2µÄHClČÜŅŗÖŠ£¬ÓÉĖ®µēĄė³öµÄH+µÄŹżÄæ0.01NA | |
| B£® | ±ź×¼×“æöĻĀ£¬2.24LµÄCCl4ÖŠŗ¬ÓŠµÄĀČŌ×ÓŹżĪŖ0.4NA | |
| C£® | ³£ĪĀĻĀ£¬1molCO2ÖŠŗ¬ÓŠµÄ¹²ÓƵē×Ó¶ŌŹżÄæĪŖ2NA | |
| D£® | ±ź×¼×“æöĻĀ£¬2.24L Cl2ÓėĖ®³ä·Ö·“Ó¦£¬×ŖŅʵĵē×ÓŹżŠ”ÓŚ0.1NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

| ČŪµć/”ę | ·Šµć/”ę | ±ø×¢ | |
| °×Į× | 44 | 280.5 | |
| PH3 | -133.8 | -87.8 | ÄŃČÜÓŚĖ®”¢ÓŠ»¹ŌŠŌ |
| SiF4 | -90 | -86 | Ņ×Ė®½ā |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com