
˫��ˮ(H2O2)��һ�ּ�������ʣ�Ҳ��һ�֡���ɫ����������Ϊ�����桢����ķ��㣬��ҵ�Ͻ���ת��Ϊ��̬��̼����(2Na2CO4��3H2O)�������ʾ���Na2CO3��H2O2��˫�����ʡ���ش��������⣺
��1��д��H2O2�ĵ���ʽ�� ����д������SO2��Ӧ�Ļ�ѧ����ʽ ��
��2������H2O2���ɶ�Ԫ���ᣬд������ˮ�е�һ������ķ���ʽ ��
��3���������ʲ���ʹ��̼����ʧЧ����
A��MnO2 B��NaHCO3 C��H2S D��CH3COOH
��4��ϡH2SO4����Cu��Ӧ������ϡH2SO4�м���H2O2�����ʹCu�ܽ⣬д���÷�Ӧ�Ļ�ѧ����ʽ�����õ����ű�������ת�Ƶķ������Ŀ��
��5��H2O2��Ϊ����ɫ���������������� ��
 һ����������ϵ�д�
һ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)����H2O2�����Ƕ�Ԫ���ᣬ��д������ˮ�еĵ��뷽��ʽ________________________��
(2)ˮ��������H3O+��OH-����ˮ����ż���롣ͬˮһ����H2O2Ҳ�м�������ż���룬����ż����ķ���ʽΪ________________________________________��
(3)�������ʲ���ʹ��̼����ʧЧ����( )
A.MnO2 B.NaHCO
(4)ϡ�����ͭ��Ӧ������ϡ�����м���H2O2�����ʹͭ˳���ܽ⣬д���÷�Ӧ�Ļ�ѧ����ʽ����������ת�Ƶķ������Ŀ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���㽭ʡ��һ�и�����ѧ��10���¿�����ѧ�� ���ͣ������
˫��ˮ(H2O2)��һ�ּ�������ʣ�Ҳ��һ�֡���ɫ����������Ϊ�����桢����ķ��㣬��ҵ�Ͻ���ת��Ϊ��̬��̼����(2Na2CO4��3H2O)�������ʾ���Na2CO3��H2O2��˫�����ʡ���ش��������⣺
��1��д��H2O2�ĵ���ʽ�� ����д������SO2��Ӧ�Ļ�ѧ����ʽ ��
��2������H2O2���ɶ�Ԫ���ᣬд������ˮ�е�һ������ķ���ʽ ��
��3���������ʲ���ʹ��̼����ʧЧ����
| A��MnO2 | B��NaHCO3 | C��H2S | D��CH3COOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ�����йٶ������и�һ9���¿���ѧ�Ծ����������� ���ͣ�ʵ����
��14�֣���������װ��ͼ�ش�����(װ��ͼ�÷��ű�ʾ)��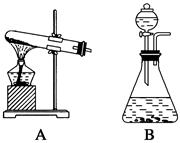
(1)˫��ˮ(H2O2)����ɫҺ�壬�ɷ������»�ѧ��Ӧ��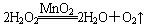 ����Ӧ��MnO2��������________����ʵ�������ô˷�Ӧ��ȡ����ʱ��Ӧѡ�õ����巢��װ����________��������鱾װ�������Եķ�����______________________________________________��
����Ӧ��MnO2��������________����ʵ�������ô˷�Ӧ��ȡ����ʱ��Ӧѡ�õ����巢��װ����________��������鱾װ�������Եķ�����______________________________________________��
(2)KClO3��MnO2������ʱ������Ҳ�����Ƶ�������Ӧѡ�õ����巢��װ����________��
(3)Ϊ����֤MnO2��KClO3�ֽ���������˴������ã�����Ҫ�ѷ�Ӧ��IJ�����룬��ȡ��MnO2����֤���ȷ�Ǵ����������MnO2�IJ�����________��________��ϴ�ӡ���ɡ�������Ϊ֤��MnO2�Ǵ���������Ҫ֪����һ��������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ�����и�һ9���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
��14�֣���������װ��ͼ�ش�����(װ��ͼ�÷��ű�ʾ)��
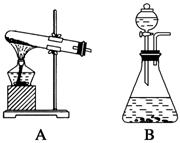
(1)˫��ˮ(H2O2)����ɫҺ�壬�ɷ������»�ѧ��Ӧ��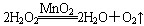 ����Ӧ��MnO2��������________����ʵ�������ô˷�Ӧ��ȡ����ʱ��Ӧѡ�õ����巢��װ����________��������鱾װ�������Եķ�����______________________________________________��
����Ӧ��MnO2��������________����ʵ�������ô˷�Ӧ��ȡ����ʱ��Ӧѡ�õ����巢��װ����________��������鱾װ�������Եķ�����______________________________________________��
(2)KClO3��MnO2������ʱ������Ҳ�����Ƶ�������Ӧѡ�õ����巢��װ����________��
(3)Ϊ����֤MnO2��KClO3�ֽ���������˴������ã�����Ҫ�ѷ�Ӧ��IJ�����룬��ȡ��MnO2����֤���ȷ�Ǵ����������MnO2�IJ�����________��________��ϴ�ӡ���ɡ�������Ϊ֤��MnO2�Ǵ���������Ҫ֪����һ��������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���㽭ʡ������ѧ��10���¿�����ѧ�� ���ͣ������
˫��ˮ(H2O2)��һ�ּ�������ʣ�Ҳ��һ�֡���ɫ����������Ϊ�����桢����ķ��㣬��ҵ�Ͻ���ת��Ϊ��̬��̼����(2Na2CO4��3H2O)�������ʾ���Na2CO3��H2O2��˫�����ʡ���ش��������⣺
��1��д��H2O2�ĵ���ʽ�� ����д������SO2��Ӧ�Ļ�ѧ����ʽ ��
��2������H2O2���ɶ�Ԫ���ᣬд������ˮ�е�һ������ķ���ʽ ��
��3���������ʲ���ʹ��̼����ʧЧ����
A��MnO2 B��NaHCO3 C��H2S D��CH3COOH
��4��ϡH2SO4����Cu��Ӧ������ϡH2SO4�м���H2O2�����ʹCu�ܽ⣬д���÷�Ӧ�Ļ�ѧ����ʽ�����õ����ű�������ת�Ƶķ������Ŀ��
��5��H2O2��Ϊ����ɫ���������������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com