
ijѧϰС��������ͼ��ʾװ�òⶨ��þ�Ͻ������������������������ԭ��������

��1��A���Լ�Ϊ__________��
��2��ʵ��ǰ���Ƚ���þ�Ͻ���ϡ���н���Ƭ�̣���Ŀ����____________________��
��3����������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú�����еIJ������У��ټ�¼C��Һ��λ�ã��ڽ�B��ʣ�������ˣ�ϴ�ӣ�������أ��۴�B�в���������������ָ������º�¼C��Һ��λ�ã�����A��B�μ������Լ����ݼ�������ԡ�����������˳����__________������ţ�����¼C��Һ��λ��ʱ��������ƽ���⣬��Ӧ__________��
��4��B�з�����Ӧ�Ļ�ѧ����ʽΪ____________________��
��5����ʵ������þ�Ͻ������Ϊa g������������Ϊb mL���ѻ���Ϊ��״������B��ʣ����������Ϊc g?�����������ԭ������Ϊ__________��
��6��ʵ������У���δϴ�ӹ������õIJ�������õ�����������__________���ƫ��ƫС������Ӱ�족����
��1��NaOH��Һ
��2����ȥ��þ�Ͻ���������Ĥ
��3���ݢ٢ܢۢڡ�ʹD��C��Һ����ƽ
��4��2Al��2NaOH��2H2O ==== 2NaAlO2��3H2��
��5��![]() ����6��ƫС
����6��ƫС
��1���۲�ʵ��װ��ͼ��֪�������������ʵ��Ŀ�ģ���A��ӦʢNaOH��Һ����B�з���2Al��2NaOH��2H2O ==== 2NaAlO2��3H2���ķ�Ӧ��
��2��Ŀ���dz�ȥ��þ�Ͻ���������Ĥ��
��3����ȷ˳��ӦΪ�ݢ٢ܢۢڣ�ΪʹC�������ѹǿ��������ѹ��ȣ���ӦʹD��C��Һ����ƽ��
��5����
2Al �� 3H2
2 mol 3 mol
![]() ?
? ![]()
��M��Al��=![]() ��
��
��6����w��Al��=![]() ��100%�ļ��㹫ʽ��֪δϴ�Ӳ���������������������ƫС��
��100%�ļ��㹫ʽ��֪δϴ�Ӳ���������������������ƫС��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
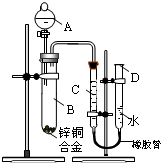 ijѧϰС��������ͼ��ʾװ�òⶨпͭ�Ͻ���п��ͭ������������
ijѧϰС��������ͼ��ʾװ�òⶨпͭ�Ͻ���п��ͭ�������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�̲�����һ����ʾʵ�飺����֬�ް�סԼ0.2 g Na![]() O
O![]() ��ĩ������ʯ�����ϣ��ɹ۲쵽��֬��ȼ��������
��ĩ������ʯ�����ϣ��ɹ۲쵽��֬��ȼ��������
��1����ʵ�������ܵó����й�Na![]() O
O![]() ��ˮ��Ӧ�Ľ����ǣ�a.���������ɣ�b.____________��
��ˮ��Ӧ�Ľ����ǣ�a.���������ɣ�b.____________��
��2��ij�о���ѧϰС����������ͼ��ʾװ�ý���ʵ�飬��֤���������ۡ�
��������֤����a��ʵ�鷽����������____________________________��
��������֤����b��ʵ�鷽����������____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com