
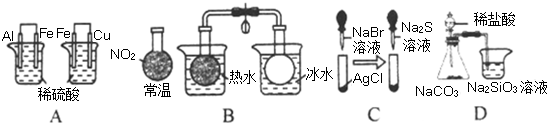
�о���ѧϰС��Ϊ̽��Cu��ŨH
2SO
4��Ӧ�������SO
2�����ʣ��������ʵ��װ�ã�
��1��д��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ��
Cu+2H
2SO
4��Ũ��
CuSO
4+SO
2+2H
2O
Cu+2H
2SO
4��Ũ��
CuSO
4+SO
2+2H
2O
�����м�Һ�����������ǣ�
����SO2��ֹ��Ⱦ����
����SO2��ֹ��Ⱦ����
��
��2����С��ͬѧ��ʵ���з�������ʵ��װ�����൱���֮������ʵ�鲻����ȫ������ɻ�����Ⱦ�ȣ�Ϊ�Ľ�ʵ�������˽�SO
2�����ʣ�����ͬѧ������ۺ�����ʦ�Ľ������������ͼʵ��װ�ã�

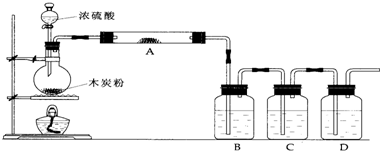
�ٶ��Թ�A�е�ŨH
2SO
4��ͭ˿���м��ȣ�����E�Թ����������ݳ���Ʒ����Һ �ܿ���ɫ�������� δ��D�Թ�������������Һ���ֻ��ǣ�Ϊ̽��D�Թ���δ���ֻ��ǵ�ԭ��С��ͬѧ�ڻ�ѧ�ֲ���ֻ���ĵ��������ʳ����µ��ܽ�����ݣ�
| ���� |
�ܽ�ȣ�g/100ˮ�� |
���� |
�ܽ�ȣ�g/100ˮ�� |
| Ca��OH��2 |
0.173 |
Ba��OH��2 |
3.89 |
| CaCO3 |
0.0013 |
BaSO3 |
0.016 |
| Ca��HCO3��2 |
16.60 |
|
|
����Ӧ������о�����Ԥ����D�Թ�δ���ֻ��ǵ�ԭ��
�������ܽ�Ƚϴ��Ba��HSO3��2
�������ܽ�Ƚϴ��Ba��HSO3��2
��
��Ϊ��֤D�Թ�����Һ����ɣ�����������ʵ�飬������������ʵ�鱨�森
| ʵ�鷽�� |
���� |
| 1ȡ������Һ���Թ��У���������������Һ |
|
| 2ȡ������Һ���Թ��У�����ϡ���ᣬ���ȣ���ʪ�����ɫʯ����ֽ�������ɵ����壮 |
|
��3��ʵ����������Թ�A�л���ͭƬʣ�࣮��С���ͬѧ������ѧ�Ļ�ѧ֪ʶ��Ϊ����Ҳ��ʣ�࣮����ҩƷ���ܹ�����֤����Ӧ��������Թ�A��ȷ���������
A D
A D
����д��ĸ��ţ���
A������ B���Ȼ�����Һ C������ D��̼��������Һ
��4�����������B������
��ֹC�е�Һ�嵹��������鷴Ӧʱ�����Ƿ�������жװ��ǰ��B�ܿ����Թ�A�д������������
���ž�A�еĶ����������壬���������Ⱦ�ȣ�
��ֹC�е�Һ�嵹��������鷴Ӧʱ�����Ƿ�������жװ��ǰ��B�ܿ����Թ�A�д������������
���ž�A�еĶ����������壬���������Ⱦ�ȣ�
��ֻдһ��Ϳɣ���



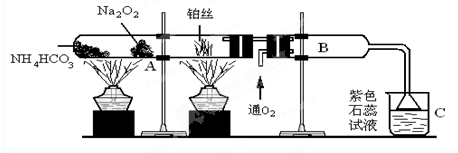
 ��������ϵ�д�
��������ϵ�д� ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�
 �о���ѧϰС��Ϊ̽��Cu��ŨH2SO4��Ӧ�������SO2�����ʣ��������ʵ��װ�ã�
�о���ѧϰС��Ϊ̽��Cu��ŨH2SO4��Ӧ�������SO2�����ʣ��������ʵ��װ�ã�
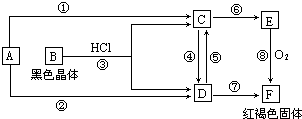
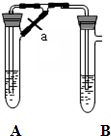 �ס�����ͬѧ����ȡ������Fe��OH��2��������ͼ��ʾ��װ�ý������飮A������Fe��ϡ���ᣬB������NaOH��Һ���ش��������⣮
�ס�����ͬѧ����ȡ������Fe��OH��2��������ͼ��ʾ��װ�ý������飮A������Fe��ϡ���ᣬB������NaOH��Һ���ش��������⣮