
������������ȷ����
��1��������̼�ظ�������
��2���ð�ˮ��ȥ�Թ��ϵ�����
��3�������������ά������̫���ܵ�ص���Ҫԭ��
��4������ϡ���ᡢ̼������Һ����������Һ���ʵ������֤Ԫ�صķǽ�����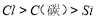
��5������ֻ�ܸı䷴Ӧ�Ļ�ܣ����ܸı䷴Ӧ����ЧӦ
��6����������̲������������������������跴Ӧ������
��7��ͬ����Ԫ�صļ������ӻ�ԭ��Խǿ��ˮ��̶�Խ��
��8��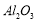 �ڹ�ҵ�������������²��ϣ�Ҳ���ڵ�ⷨ������
�ڹ�ҵ�������������²��ϣ�Ҳ���ڵ�ⷨ������
��9�������ЧӦ������������Һ�뽺�壬�ơ������ܲ��������ЧӦ
��10������������ˮ��������ɱ����̼�ᱵ�����ڱ�����
A����1����4����6����7�� B����4����6����9����10��
C����3����5����6����8�� D����5����6����8����9��
D
��������
�����������1��̼�ظ����γ�ԭ��أ������⣬��̼�ظֱȴ��������⣬�ʴ���2����ˮ����������Ӧ������������ϴ���������ʴ���3�������������������ά��ԭ�ϣ���Si������̫���ܵ�ص���Ҫԭ�ϣ��ʴ���4�������ӷ����������������Һ��Ӧ���ɹ��ᣬ����ȷ��̼�����������ԣ��ʴ���5�������ı䷴Ӧ�Ĺ��̣����ı�ʼ��̬�����Դ���ֻ�ܸı䷴Ӧ�Ļ�ܣ����ܸı䷴Ӧ����ЧӦ������ȷ����6�����������������跴Ӧ�����ķ����衢������������������̲���������ȷ����7��ͬ����Ԫ�صļ������ӻ�ԭ��Խǿ����ǽ�����Խ������ˮ���أ���ڢ����������Ӳ�ˮ�⣨F-���⣩���ʴ���8��Al2O3���۵�ߣ��ڹ�ҵ�������������²��ϣ�Ϊ���ӻ����Ҳ���ڵ�ⷨ������������ȷ����9�������ЧӦΪ�������е����ʣ�������������Һ�뽺�壬�ơ������ڽ��壬���ܲ��������ЧӦ������ȷ����10��������������ˮ�⣬�ɾ���ˮ��������ǿ�����ԣ�����ɱ��������̼�ᱵ�����ᷴӦ�����ɱ������ж���Ӧѡ���ᱵ�����ͣ��ʴ���ѡD��
���㣺���鳣��Ԫ�ؼ��仯�������ʡ���;���й��ж�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�����и�����ѧ�����л�ѧ�Ծ��������棩 ���ͣ������
[��ѧһѡ��2����ѧ�뼼��]��15�֣�
�Ȼ���ͭ(CuCl)�ǰ�ɫ��ĩ������ˮ���������Ҵ����ڿ����лᱻѸ����������ɫ��ʽ�Ρ������Ե�Ʒ�Һ(��Ҫ��Cu2����Fe3��)���Ʊ��Ȼ���ͭ�Ĺ�������ͼ���£�

�������Ӻ�������ҺpH��CuCl��������ҺpH�Ĺ�ϵͼ��ͼ��

����֪����������Ũ��Ϊ1 mol��L��1ʱ��Fe(OH)3��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ1.4��3.0��Cu(OH)2��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ4.2��6.7����ش��������⣺
��1�����ʱ������Ӧ�����ӷ���ʽ��________������CuCl����ʱ�����pH��________���ҡ�
��2�����ۡ��Ȼ��ơ�����ͭ����Һ�з�Ӧ����CuCl�����ӷ�Ӧ����ʽΪ____________________________��
��3��������CuCl����Ҫ��������ˮ�Ҵ�ϴ�ӣ�����ո��������70�����2 h����ȴ�ܷ��װ��70����ո���ܷ��װ��Ŀ����____________________________________________��
��4����Ʒ�˳�ʱ������Һ����Ҫ�ֳ���________���������Һ�л�ȡFeSO4��7H2O���壬����Ҫ֪������__________________��
��5���������ۻ�����������Ҳ�ɵõ��Ȼ���ͭ����д���÷�Ӧ�Ļ�ѧ����ʽ��______________________��Ϊ���CuCl�IJ��ʣ����ڸ÷�Ӧ��ϵ�м���ϡ����Һ������pH��3.5����������Ŀ����__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ���㽭ʡ����У������ѧ������������ѧ�Ծ��������棩 ���ͣ�ѡ����
�����ᣨHN3��������������ƣ����������д������
A��HN3ˮ��Һ����Ũ�ȴ�С˳��Ϊ��c(HN3)>c(H+)>c(N3-)>c(OH-)
B��HN3��NH3�������ɵĵ��������ȫ���ǹ��ۼ�
C��NaN3ˮ��Һ������Ũ�ȴ�С˳��Ϊ��c(Na+)>c(N3-) >c(OH-)>c(H+)
D��N3-��CO2����ȵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ������ѧ�ڵڶ����¿���ѧ�Ծ��������棩 ���ͣ������
����ѧ����ѡ��2����ѧ�뼼������15�֣�
�����ķ�չ�봴������ֹ�����ҹ���ѧ�Һ�°�ĸ����Ĵ����������գ��������̿ɼ�Ҫ��ʾ��ͼ

��1�����������Ҫͨ��CO2�Ͱ�����Ӧѡͨ��______(�ѧʽ)��ԭ����______________��
��2���������з�����Ӧ�Ļ�ѧ����ʽ��__________________________________��
��3��ĸҺ�е�������Ҫ��________����ĸҺ��ͨ��������ϸСʳ�ο�������ȴ��������Ʒ��ͨ�백����������_____________________________________________��
��4��ʹԭ���Ȼ��Ƶ������ʴ�70%��ߵ�90%���ϣ���Ҫ�������________(�����������еı��)��ѭ��������X��__________���ӳ���������ȡ�����IJ�����__________��
��5��д������¯�з�����Ӧ�Ļ�ѧ����ʽ_____________________________��
��6�������ƵõIJ�Ʒ̼�����п��ܺ��е�������____________(�ѧʽ)��Ϊ������� �ʵĴ��ڣ����������__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ������ѧ�ڵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪W��X��Y��ZΪ������Ԫ�أ�W��Zͬ���壬X��Y��Zͬ���ڣ�W����̬�⻯����ȶ��Դ���Z����̬�⻯����ȶ��ԣ�X��YΪ����Ԫ�أ�X�������ӵ�������С��Y�������ӵ������ԡ�����˵����ȷ����
A��W����̬�⻯��ķе�һ������Z����̬�⻯��ķе�
B��W��X�γɵĻ�������ֻ�����Ӽ�
C��X��Y��Z��W��ԭ�Ӱ뾶���μ�С
D����W��Yԭ���������5��������γɻ�����Ļ�ѧʽһ��ΪY2W3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ������ѧ�ڵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£������и�����������Һ�У�һ���ܴ����������������
A����ʹpH��ֽ�ʺ�ɫ����Һ��Na����NH4+��I����NO3��
B��������������H2����Һ��K����Mg2����SO42����HCO3��
C��c(Fe3��)��0.1mol��L��1����Һ��H����Al3����I����SCN��
D�� ��0.1mol��L��1����Һ��Na����K����SiO32����NO3��
��0.1mol��L��1����Һ��Na����K����SiO32����NO3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ������ѧ�ڵڶ���ģ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��12�֣����������һ�ֵ��͵�ǿ��������������ʵ���һ����ڻ��������ж�����Ҫ��Ӧ�á���ͼK10?3��ʵ�����Ʊ�����������һϵ�����ʵ���װ��(�г��豸����)��

ͼK10?3
��1���Ʊ�����ѡ�õ�ҩƷΪ������غ�Ũ���ᣬ��Ӧ�����ӷ���ʽΪ___________________________��
��2��װ��B��������________________________��ʵ�����ʱC�п��ܷ�����������д����������ʱB�е�����______________________________________��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η���____(ѡ��a����b����c��)��
a | b | c | |
I | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
�� | ��ʯ�� | Ũ���� | ��ˮ�Ȼ��� |
�� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��4�����װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ�����D�л���ͨ����������ʱ�����Կ�����ɫ��Һ��Ϊ����ɫ��˵���ȵķǽ����Դ����塣��������D�е�������Һ����E�У���E���۲쵽��������________________________________��������________(��ܡ����ܡ�)˵����ķǽ�����ǿ�ڵ⡣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ������ѧ�ڵڶ���ģ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
������װ�ò��ܴﵽ�й�ʵ��Ŀ�ĵ���

A���ü�ͼװ��֤����(ú��)<��(��)<��(ˮ)
B������ͼװ���Ʊ�Fe(OH)2
C���ñ�ͼװ����ȡ������
D���ö�ͼװ�ñȽ�NaHCO3��Na2CO3�����ȶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꽭��ʡ������ʮ���ظ�����ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪����K2Cr2O7��Һ����FeSO4��Ӧ����Fe3+��Cr3+���ֽ������ữ��K2Cr2O7��Һ��FeSO4��Һ��ϣ���ַ�Ӧ������������Һ�м���KI��Һ,�����Һ��Fe3+�����ʵ���������KI�����ʵ����ı仯��ϵ��ͼ��ʾ��
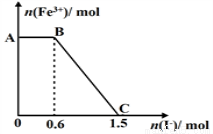
����˵���в���ȷ����
A��ͼ��AB�ε�������ΪK2Cr2O7
B��ͼ��BC�η����ķ�ӦΪ2Fe3++2I- =2Fe2++I2
C����ʼ�����K2Cr2O7Ϊ0.25 mol
D��K2Cr2O7����FeSO4��Ӧ�����ʵ���Ϊ1��3��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com