
���� ��1���ٸ���������Һ��һ��ʵ���������ȷ��ÿ��������Ҫ������
�����ݲ�������ϡ�ͺ���Һ���������ý��
������ƿ���л�����ʹ�ù�������Ҫ���µߵ�������ʹ��ǰ�������Ƿ�©ˮ��
��������Ͳ������ƿ���켰ʹ��ע��������
�ݴ����������ʵ��ʧ���Ҳ��ܲ��ȵı����������ƣ�
��2����Һ���Ʋ�������Ͳ�н��У�Ũ����ϡ�����в����������ȣ�����ϡ��Ũ����Ӧ��Ũ���Ỻ��ע��ˮ�У�
��� �⣺��1����ʵ��������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢��������ȡ�����ܽ⣨ϡ�ͣ�����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ�����������Ͳ���ձ�������������ͷ�ιܡ�100mL����ƿ�����Ի�ȱ�ٵ�������100mL����ƿ��
�ʴ�Ϊ��100mL����ƿ��
������һ�����ʵ���Ũ����Һʱ����������ϡ���������ý�������ܽ⣬����Һ����������������
�ʴ�Ϊ�������ܽ⣬������
������ƿ���л�����ʹ�ù�������Ҫ���µߵ�������ʹ��ǰ�������Ƿ�©ˮ��
�ʴ�Ϊ������Ƿ�©Һ��
��a��ϴ����ȡŨH2SO4�����Ͳ��������ȡ��Ũ�������ƫ������ʵ������a����
b������ƿΪ������������ҺǰҺ��Ӧ��ȴ�����£���b��ȷ��
c��ת��ǰӦ��ʹ�õ�����ƿ����Ҫ�����Ϊ����ʱ����Ҫ��������ˮ����ʵ������Ӱ�죬��c����
d������ҡ�Ⱥ��ְ�Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�����ˮ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫС����d����
��ѡ��b��
��������ʱ��С��ʹҺ�泬���˿̶��ߣ�����ʵ��ʧ�ܣ��Ҳ��ܲ��ȣ�Ӧ��ȡ�Ĵ�ʩ�������ƣ�
�ʴ�Ϊ���������ƣ�
��2����Һ���Ʋ�������Ͳ�н��У�Ũ����ϡ�����в����������ȣ�����ϡ��Ũ����Ӧ��Ũ���Ỻ��ע��ˮ�У����Ըò�������������������������Ͳ������Һ�����ܽ�ˮ���뵽Ũ�����У�
�ʴ�Ϊ����������Ͳ������Һ�����ܽ�ˮ���뵽Ũ�����У�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ�������Ʋ��������ǽ���ؼ���ע������ƿ���켰ʹ�÷�����ע��Ũ����ϡ�͵���ȷ������������Ŀ�ѶȲ���


 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȩ���� | B�� | Cu��OH��2���� | ||
| C�� | ��CuSO4��ȡCu��OH��2ʱ��NaOHҪ���� | D�� | ��CuSO4��ȡCu��OH��2ʱ��CuSO4Ҫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������������ѧ�߶��ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���ڿ��淴Ӧ2AB3��g�� 2A��g��+3B2��g����H��0 ����ͼ����ȷ����
2A��g��+3B2��g����H��0 ����ͼ����ȷ����
A�� B��
B��
C��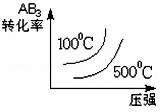 D��
D��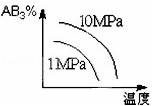
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������и�һ��9�µ��л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��4��ʱ��100mLˮ���ܽ���22.4L HCl���壨��״���²�ã����γɵ���Һ������˵������ȷ����
A������Һ���ʵ���Ũ��Ϊ10m ol��L��1
ol��L��1
B������Һ���ʵ���Ũ������Һ���ܶ�δ֪�������
C������Һ�����ʵ�������������Һ���ܶ�δ֪�������
D��������Һ�����Ϊ22.5L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �ս̰桶��ѧ1�������̽����������100mL 0.100mol•L-1 Na2CO3��Һ��
�ս̰桶��ѧ1�������̽����������100mL 0.100mol•L-1 Na2CO3��Һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ | |
| B�� | �κη��ȷ�Ӧ�ڳ���������һ���ܷ�����Ӧ | |
| C�� | ��Ӧ��������������е������������˷��Ȼ������� | |
| D�� | ���ȷ�Ӧֻ���ڼ��ȵ������²��ܽ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com