
����Ŀ����(34Se)����ͬ���壬��Ԫ�ؼ��仯���������彡������ҵ����������ء�ij����С����������(��Ҫ�ɷ���Se������CuSe��Ag2Se������)Ϊԭ�ϣ����������������£�

��ش��������⣺
(1)��ԭ�ӵĴ���������_______,����ͬ��������Ԫ����________(��Ԫ������)��
(2)��֪A��Na2SeO3�����������ƿ�ɽ��,��A�Ļ�ѧ����Ϊ______��C��Na2Se����Na2Se�ĵ���ʽΪ_______��
(3)��������ͼ�е������ڡ�( )�����������Ⱥ�˳��������д��������_____��_____��
(4)д���������ý�̿��ԭB�Ļ�ѧ����ʽ___________________��
(5)��ҺC�������������ӷ���ʽ____________________��
(6)��Na2SeO3��Һ�еμ��Թ��������ᣬ�����ӷ���ʽΪ__________________����֪:Ka1(H2SeO3)=2.7��10-3��Ka2(H2SeO3)=2.5��10-8��Ka(CH3COOH)=1.8��10-5��
(7)�����ɲ����������ķ��������ᴿ,��ô������������Ļӷ��������������¶ȵĹ�ϵ��ͼ��ʾ��

��������п��Ƶ�����¶���________(����)��
A.455�� B.462�� C.475�� D.515��
���𰸡� 18 �顢�� �������� ![]() ���� ���� Na2SeO4+4C
���� ���� Na2SeO4+4C![]() Na2Se+4CO�� 2Se2-+O2+2CO2=2Se��+2CO32-(��2Se2-+O2+4CO2+2H2O=2Se��+4HCO3-) SeO32-+CH3COOH=HSeO3-+CH3COO- C
Na2Se+4CO�� 2Se2-+O2+2CO2=2Se��+2CO32-(��2Se2-+O2+4CO2+2H2O=2Se��+4HCO3-) SeO32-+CH3COOH=HSeO3-+CH3COO- C
��������(1). ��֪������ͬ���壬���������Ϊ34����Ԫ�ص��������6�����ӣ�K����2�����ӣ�L����8�����ӣ���M����18�����ӣ�ͬ�������������ڵ�Ԫ��������壬�ʴ�Ϊ��18���顢�壻
(2). ���Na2SO3�Ļ�ѧ�������������ƿ�֪��Na2SeO3�Ļ�ѧ�������������ƣ�SeԪ���������6�����ӣ��õ�2��Naԭ��ʧȥ��2�������γ��ȶ��ṹ��Na2Se�ĵ���ʽΪ��![]() ���ʴ�Ϊ���������ƣ�
���ʴ�Ϊ���������ƣ�![]() ��
��
(3). ��ˮ��֮ǰӦ���ս��Ĺ�����飬����߽����ʣ����ݡ�Cu��Ag����������ҺA�����ж�ˮ����IJ����ǹ��ˣ��ʴ�Ϊ�����飻���ˣ�
(4). A��Na2SeO3��ͨ����������ɣ��õ��Ĺ���B��Na2SeO4���������ý�̿��ԭNa2SeO4�õ�Na2Se��CO�����ݵ�ʧ�����غ��ԭ���غ㣬�÷�Ӧ�Ļ�ѧ����ʽΪ��Na2SeO4+4C![]() Na2Se+4CO�����ʴ�Ϊ��Na2SeO4+4C
Na2Se+4CO�����ʴ�Ϊ��Na2SeO4+4C![]() Na2Se+4CO����
Na2Se+4CO����
(5). ����ҺC��ͨ������������ɰѻ�ԭ��ǿ��Se2-����Ϊ����Se��ͨ��CO2���Լ�����Ӧ����Һ�ļ��ԣ�������Se���������������֪�÷�Ӧ�����ӷ���ʽΪ��2Se2-+O2+2CO2=2Se��+2CO32-(��2Se2-+O2+4CO2+2H2O=2Se��+4HCO3-)���ʴ�Ϊ��2Se2-+O2+2CO2=2Se��+2CO32-(��2Se2-+O2+4CO2+2H2O=2Se��+4HCO3-)��
(6). ��H2SeO3��CH3COOH�ĵ��볣����֪������ǿ����˳��Ϊ��H2SeO3��CH3COOH��HSeO3��������Na2SeO3��Һ�еμ��Թ��������ᣬ��Ӧ����HSeO3����CH3COO�������ӷ���ʽΪ��SeO32-+CH3COOH=HSeO3��+CH3COO�����ʴ�Ϊ��SeO32-+CH3COOH=HSeO3��+CH3COO����
(7). ��ͼ��֪����475��ʱ�������Ļӷ���������������������ѡ��475�棬�ʴ�Ϊ��C��


 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼװ��ģ���Ʊ�������(��ѧʽΪCa3N2��������H2O��Ӧ)������˵������ȷ����

A. ����Kͨ��N2���Թ�A�������ݲ�����˵��װ������������
B. U�ι���ʢ�ŵĸ���������Ǽ�ʯ�ң���������Ũ����
C. ��Ӧ��������Ϩ��ƾ��ƣ�����Ӧ����ȴ�����º��ٹرջ���K
D. ������Ca3N2���������У��ܵõ�CaCl2��NH4C1������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ϯ�����Ҫ��ָ�����ǰ�����ǰ����Ի���(-RC=N-)��һ���л�������������л��ϳ��Լ���Һ�����ϡ�ͨ��ϯ������ɰ��ͻ����ʻ����϶��ɡ�ijϯ��������G��һ�ֺϳ�·�����£�

��֪������Ϣ
��
��lmol B��������Ӧ������2mol C����C�ܷ���������Ӧ
��D���ڵ�ȡ������������Է�������Ϊ92
�ܺ˴Ź���������ʾF�����������ֻ�ѧ��������
��
�ش���������
��1����A����B�Ļ�ѧ����ʽΪ_____________����Ӧ����Ϊ_______________��
��2��D��������____________����D����E�Ļ�ѧ����ʽΪ_______________��
��3��G�Ľṹ��ʽΪ_______________��
��4��C8H11N��ͬ���칹���к��б����Ĺ���_____��(�����������칹)�����к˴Ź�������Ϊ4��壬�������Ϊ6��2��2��1����___________(д������һ�ֵĽṹ��ʽ)��
��5�������ϳ�·�ߣ����һ���ɱ���������C�ϳ��һ�����(![]() )�ĺϳ�·�ߣ�_________________________
)�ĺϳ�·�ߣ�_________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ԭCO2�ǽ������ЧӦ����Դ�������Ҫ�ֶ�֮һ���ں����ܱ������У�CO2��H2�ڴ��������·�����Ӧ��CO2(g) + 3H2(g) ![]() CH3OH(g) + H2O(g)��CO2��H2��CH3OH��H2O��Ũ�Ⱦ����ٸı�ʱ������˵����ȷ����
CH3OH(g) + H2O(g)��CO2��H2��CH3OH��H2O��Ũ�Ⱦ����ٸı�ʱ������˵����ȷ����
A. CO2��H2��CH3OH��H2O��Ũ��һ�����
B. �÷�Ӧ�Ѿ��ﵽ��ѧƽ��״̬
C. CO2��H2��ȫת��ΪCH3OH��H2O
D. CO2��H2�ķ�Ӧ���ʵ���CH3OH��H2O�ķ�Ӧ������Ϊ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����г��ø��ϼ���ȡ���࣬�����Լ���RMgX�����Ʒ��ǣ�RX+Mg ![]() RMgX��RΪ������XΪ±�أ������Լ��ɷ�������ת�䣺
RMgX��RΪ������XΪ±�أ������Լ��ɷ�������ת�䣺 
��R��R���������ͬ��ͬ��������
��AΪԭ�Ϻϳ������춡���� ![]() �����������£����ַ�Ӧ�P��Ӧ����û���г�����A��Ҫ��Դ��ʯ���ѽ�����A�IJ�������Ϊ����ʯ�ͻ���ˮƽ�ı�־��
�����������£����ַ�Ӧ�P��Ӧ����û���г�����A��Ҫ��Դ��ʯ���ѽ�����A�IJ�������Ϊ����ʯ�ͻ���ˮƽ�ı�־��
�Իش�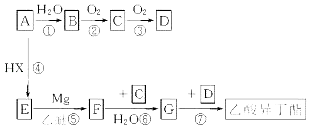
��1�����������У����ڻӳɷ�Ӧ���ǣ���д��ţ� ��
��2��д��F�Ľṹ��ʽ ��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ����Ӧ�� �� ��Ӧ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʯ��ʯ��ʳ�Ρ���̿��ˮΪԭ�ϣ�д���ϳɾ�����ϩ�Ļ�ѧ����ʽ������֪��CaO+3C ![]() CaC2+CO����
CaC2+CO����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��D��E Ϊԭ���������������ǰ������Ԫ�ء���֪ǰ����Ԫ�صĻ�̬ԭ��p�ܼ�����2�������ӣ�E ��ԭ����������A��B��C����Ԫ��ԭ������֮�͡�
�Իش���������:
(1)��̬Eԭ����Χ�����Ų�ͼΪ_________������M �ܲ���_____��������ͬ�ĵ��ӡ�
(2)��ԭ�ӽṹ�ǶȽ���B �縺�Դ���D��ԭ����:_______________��
(3) ��AԪ�صĻ������У�A ��ԭ�Ӽ䳣�Цм������Ǻ�CԪ�صĻ������У�C��ԭ�Ӽ�ֻ�ܴ��ڦҼ�������Ҫԭ����___________________��
(4)H2D2B8��һ�־���ǿ�����ԵĶ�Ԫ��(���з��ӽṹ����2��Bԭ����-1��),��H2D2B8�ĽṹʽΪ___________�������в�ȡsp3�ӻ���Bԭ����______����
(5)E ���������ӵĶѻ�ģ����ͼ��ʾ��

��֪:E �������ܶ�Ϊ��g/cm3��NA���������ӵ�����ֵ��E �����ԭ������ΪM��
��E ���ӵ���λ��Ϊ_______��
��E ���Ӱ뾶Ϊ_______pm��
��E ���������ӵĿռ������ʦ�=_____(�ú��� ��ʽ�ӱ�ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮMgBr2 �ɹ㷺�����л���Ӧ��������ˮ�������ȡ�ʵ���ҿ���þм��Һ��Ϊԭ�ϣ�������ͼװ���Ʊ���ˮMgBr2,��ش�:
��1��ʶ����ͼ��������A��������______��B��_______
��2��ʵ��ʱ������װ��C�л���ͨ�����ĵ�����ֱ������ȫ��������ƿ�С�����ĵ����ܽ�Һ�崵������ΪҺ�����________������;ʵ���в����ø�������������N2��ԭ���ǣ�___________________________________________________________

��3����֪:Mg��Br2��Ӧ���ҷ���;����(C2H5OC2H5)���ӷ���MgBr2�������ܷ������·�Ӧ:MgBr2+3C2H5OC2H5![]() MgBr2��3C2H5OC2H5+Q(Q>0);��Ӧ��Ϻ�ָ������£����ˣ���Һת������һ�������ƿ�У���ȴ��0��C,�������壬�ٹ��˵������Ѻ��廯þ��Ʒ����һ�ι��˳�ȥ��������_______�����˱����õ��IJ���������:_______��
MgBr2��3C2H5OC2H5+Q(Q>0);��Ӧ��Ϻ�ָ������£����ˣ���Һת������һ�������ƿ�У���ȴ��0��C,�������壬�ٹ��˵������Ѻ��廯þ��Ʒ����һ�ι��˳�ȥ��������_______�����˱����õ��IJ���������:_______��
��4������ƽ���ƶ���ԭ��˵���õ������Ѻ��廯þ������������ֽ⣬����ˮMgBr2��Ʒ��ԭ��: ______________________________
��5��Ϊ�ⶨ��Ʒ�Ĵ��ȣ�����EDTA(��дΪY4-)����Һ�ζ�����Ӧ�����ӷ���ʽ:Mg2++Y4-====Mg Y2-
�ٵζ�ǰ��ϴ�ζ��ܵIJ���������____________________________
�ڲⶨǰ���ȳ�ȡ0.2500g��ˮMgBr2��Ʒ���ܽ����0.0500mol/L��EDTA ����Һ�ζ����յ㣬����EDTA ����Һ26.50mL,������ˮMgBr2��Ʒ�Ĵ�����___________ (������������ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��C��N��Si���γɶ��ָ�Ӳ�Ȳ��ϣ���Si3N4,C3N4,SiC.
(1)Si3N4��C3N4��Ӳ�Ƚϸߵ���______,������_________.
(2)C��N���γ�һ����ʯī�ṹ���ϣ���ϳɹ�������ͼ��ʾ������ʯī�ṹ���ϻ�����Ļ�ѧʽΪ_________����ϳɹ������������谷�γɣ������谷��Nԭ�ӵ��ӻ���ʽ��_____________��

(3)C��N�����γ�һ����Ԫ��״�л�������(im)����ṹΪ![]() ��������[Co(im)6]SiF6�Ľṹʾ��ͼ����:
��������[Co(im)6]SiF6�Ľṹʾ��ͼ����:
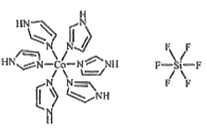
��Coԭ�ӵļ۲���ӹ������ʽ(�۲�����Ų�ͼ)Ϊ_____��N��Co֮��Ļ�ѧ��������___���жϵ�������__________��
��������SiF62-����ԭ��Si�ļ۲���Ӷ���Ϊ______��������(Co(im)6]2+��SiF62-֮������������Ӽ�ľ�����������������������ã�����������ı�ʾʽ_______��
����ˮ������ı�ʾʽΪ: ![]()
(4)SiCΪ������ϵ���壬��������Ϊa,��֪Siԭ�Ӱ뾶ΪrSi,Cԭ�Ӱ뾶ΪrC,�þ�����ԭ�ӵķ�������Ϊ:

��SiC���������к���____��Siԭ�ӡ�____��Cԭ�ӣ��þ�����ԭ�ӵ����ռ��������İٷ���Ϊ__________(�г�����ʽ����)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com