
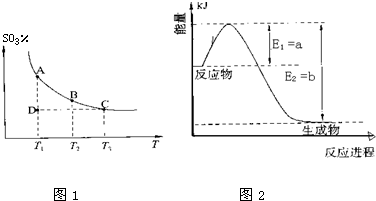
ÓŅĶ¼A”¢BŹĒµČĢå»żČŻĘ÷£¬KŹĒæŖ¹Ų£¬»īČūæÉŅŌ×óÓŅŅĘ¶Æ”£ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬¹Ų±ÕK£¬ĻņAÖŠĶØČėŅ»¶ØĮæµÄNO2”£·¢Éś£ŗ2NO2(g)  N2O4(g)£»”÷H<0”£ŌņŅŌĻĀĖµ·Ø²»ÕżČ·µÄŹĒ £Ø £©
N2O4(g)£»”÷H<0”£ŌņŅŌĻĀĖµ·Ø²»ÕżČ·µÄŹĒ £Ø £©

A£®±£³Ö»īČūĪ»ÖĆ²»±ä£¬“ņæŖK£¬ĢåĻµÖŠĘųĢåŃÕÉ«ĻȱäĒ³”¢Č»ŗóĀŌ¼ÓÉī
B£®“ņæŖKŅ»»į£¬ŌŁ¹Ų±ÕĖü£¬ĻņÓŅĶĘ¶Æ»īČūŹ±£¬×īŗóAČŻĘ÷µÄĘųĢåŃÕÉ«±ČBµÄĒ³
C£®“ņæŖKŅ»»į£¬ŌŁ¹Ų±ÕĖü£¬°ŃAČŻĘ÷¼ÓČČ£¬»īČū²»ŅĘ¶Æ£¬AÖŠĘųĢåŃÕÉ«±ČBÖŠÉī
D£®“ņæŖKŅ»»į£¬ŌŁ¹Ų±ÕĖü£¬ĻņBÖŠĶØČėė²Ęų£¬BÖŠĘųĢåŃÕÉ«²»±ä

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 CH3OH£Øg£©+H2O£Øg£©
CH3OH£Øg£©+H2O£Øg£©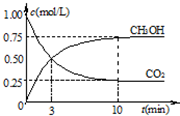 Ėꏱ¼ä±ä»ÆČēĶ¼ĖłŹ¾£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
Ėꏱ¼ä±ä»ÆČēĶ¼ĖłŹ¾£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ CH3OH£Øg£©+H2O£Øg£©“ļµ½Ę½ŗāדĢ¬µÄŹĒ
CH3OH£Øg£©+H2O£Øg£©“ļµ½Ę½ŗāדĢ¬µÄŹĒ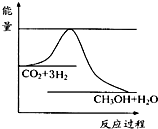
 CH3OH£Øg£©+H2O£Øg£©½ųŠŠ¹ż³ĢÖŠÄÜĮæ£Øµ„Ī»ĪŖkJ?mol-1£©µÄ±ä»Æ£®ŌŚĢå»żĪŖ1LµÄŗćČŻĆܱÕČŻĘ÷ÖŠ£¬³äČė1mol CO2ŗĶ3mol H2£¬“ļµ½Ę½ŗāŗ󣬲ÉČ”ĻĀĮŠ“ėŹ©ÖŠÄÜŹ¹c£ØCH3OH£©Ōö“óµÄŹĒ
CH3OH£Øg£©+H2O£Øg£©½ųŠŠ¹ż³ĢÖŠÄÜĮæ£Øµ„Ī»ĪŖkJ?mol-1£©µÄ±ä»Æ£®ŌŚĢå»żĪŖ1LµÄŗćČŻĆܱÕČŻĘ÷ÖŠ£¬³äČė1mol CO2ŗĶ3mol H2£¬“ļµ½Ę½ŗāŗ󣬲ÉČ”ĻĀĮŠ“ėŹ©ÖŠÄÜŹ¹c£ØCH3OH£©Ōö“óµÄŹĒ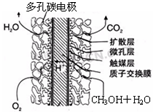
 NH3?H2O+H+
NH3?H2O+H+ NH3?H2O+H+
NH3?H2O+H+²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ||
| øßĪĀ |
| ||
| øßĪĀ |
| 4 |
| 7 |
| ||
| øßĪĀ |
| T/”ę | 200 | 300 | 400 |
| K | K1 | K2 | 0.5 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
½üÄźĄ“£¬Ėę×ÅĪŅ¹ś¾¼ĆµÄæģĖŁ·¢Õ¹£¬¶ŌµēĮ¦µÄŠčĒóŌ½Ą“Ō½øߣ¬ÕāŅ²“Ł½ųĮĖĪŅ¹śµēĮ¦¹¤ŅµøßĖŁ·¢Õ¹£¬µ«ĪŅ¹śµēĮ¦½į¹¹ÖŠ£¬»šµē±ČÖŲ·Ē³£“ó£¬Õ¼·¢µē×°»ś×ÜČŻĮæµÄ75%ŅŌÉĻ£¬ĒŅ»šµē±ČÖŲ»¹ŌŚÖšÄźÉĻÉż”£»šĮ¦·¢µē³§ŹĶ·Å³ö“óĮæµÄµŖŃõ»ÆĪļ£ØNOx£©”¢¶žŃõ»ÆĮņŗĶ¶žŃõ»ÆĢ¼µČĘųĢå»įŌģ³É»·¾³ĪŪČ¾”£¶ŌČ¼Ćŗ·ĻĘų½ųŠŠĶŃĻõ”¢ĶŃĮņŗĶĶŃĢ¼µČ“¦Ąķ£¬æÉŹµĻÖĀĢÉ«»·±£”¢½ŚÄܼõÅÅ”¢·ĻĪļĄūÓƵČÄæµÄ”£
£Ø1£©ĶŃĻõ”£ĄūÓĆ¼×Ķé“߻ƻ¹ŌNOx£ŗ
CH4(g)£«4NO2(g)===4NO(g)£«CO2(g)£«2H2O(g)£»¦¤H1£½£574kJ”¤mol£1
CH4(g)£«4NO(g)===2N2(g)£«CO2(g)£«2H2O(g)£»¦¤H2£½£1160kJ”¤mol£1
¼×ĶéÖ±½Ó½«NO2»¹ŌĪŖN2µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ ”£
£Ø2£©ĶŃĢ¼”£½«CO2×Ŗ»ÆĪŖ¼×“¼µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ
CO2(g)£«3H2(g)![]() CH3OH(g)£«H2O(g)£»¦¤H3
CH3OH(g)£«H2O(g)£»¦¤H3
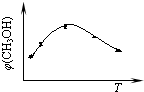
¢ŁČ”Īå·ŻµČĢå»żCO2ŗĶH2µÄ»ģŗĻĘųĢå(ĪļÖŹµÄĮæÖ®±Č¾łĪŖ1”Ć3)£¬·Ö±š¼ÓČėĪĀ¶Č²»Ķ¬”¢ČŻ»żĻąĶ¬µÄŗćČŻĆܱÕČŻĘ÷ÖŠ£¬·¢ÉśÉĻŹö·“Ó¦£¬·“Ó¦ĻąĶ¬Ź±¼äŗ󣬲āµĆ¼×“¼µÄĢå»ż·ÖŹż¦Õ(CH3OH)Óė·“Ó¦ĪĀ¶ČTµÄ¹ŲĻµĒśĻßČēÓŅĶ¼ĖłŹ¾£¬ŌņÉĻŹöCO2×Ŗ»ÆĪŖ¼×“¼µÄ·“Ó¦µÄ¦¤H3”” 0£ØĢī”°£¾”±”¢”°£¼”±»ņ”°£½”±£©”£
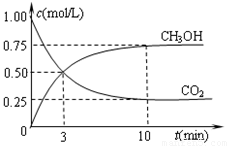
¢ŚŌŚŅ»ŗćĪĀŗćČŻĆܱÕČŻĘ÷ÖŠ³äČė1mol CO2ŗĶ3mol H2£¬½ųŠŠÉĻŹö·“Ó¦”£²āµĆCO2ŗĶCH3OH(g)µÄÅضČĖꏱ¼ä±ä»ÆČēĻĀĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ”” ””£ØĢī×ÖÄø“śŗÅ£©”£

A£®µŚ10minŗó£¬ĻņøĆČŻĘ÷ÖŠŌŁ³äČė1mol CO2ŗĶ3mol H2£¬ŌņŌŁ“Ī“ļµ½Ę½ŗāŹ±c(CH3OH)£½1.5mol”¤L£1
B£®0”«10minÄŚ£¬ĒāĘųµÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ0.075mol/(L”¤min)
C£®“ļµ½Ę½ŗāŹ±£¬ĒāĘųµÄ×Ŗ»ÆĀŹĪŖ0.75
D£®ÉżøßĪĀ¶Č½«Ź¹n(CH3OH)/n(CO2)¼õŠ”
¢Ū¼×“¼¼īŠŌČ¼ĮĻµē³Ų¹¤×÷Ź±øŗ¼«µÄµē¼«·“Ó¦Ź½æɱķŹ¾ĪŖ”” ”””£
£Ø3£©ĶŃĮņ”£Ä³ÖÖĶŃĮņ¹¤ŅÕÖŠ½«·ĻĘų¾“¦Ąķŗó£¬ÓėŅ»¶ØĮæµÄ°±Ęų”¢æÕĘų·“Ó¦£¬Éś³ÉĮņĖįļ§ŗĶĻõĖįļ§µÄ»ģŗĻĪļ×÷ĪŖø±²śĘ·»Æ·Ź”£ÉčŃĢĘųÖŠµÄSO2”¢NO2µÄĪļÖŹµÄĮæÖ®±ČĪŖ1”Ć1£¬ŌņøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ ”””£
£Ø4£©ĮņĖįļ§ŗĶĻõĖįļ§µÄĖ®ČÜŅŗµÄpH£¼7£¬ĘäÖŠŌŅņæÉÓĆŅ»øöĄė×Ó·½³ĢŹ½±ķŹ¾ĪŖ£ŗ”” ””£»ŌŚŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄĻõĖįļ§ČÜŅŗÖŠµĪ¼ÓŹŹĮæµÄNaOHČÜŅŗ£¬Ź¹ČÜŅŗµÄpH£½7£¬ŌņČÜŅŗÖŠ£ŗc(Na£«)£«c(H£«)””””c(NO)£«c(OH£)£ØĢīŠ“”°£¾”±”°£½”±»ņ”°£¼”±£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğø£½ØŹ”ĖĵŲĮłŠ£ø߶žÉĻѧʌµŚ¶ž“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ŃŠ¾æNO2”¢SO2 ”¢COµČ“óĘųĪŪČ¾ĘųĢåµÄ²āĮæ¼°“¦Ąķ¾ßÓŠÖŲŅŖŅāŅ唣
£Ø1£©I2O5æÉŹ¹H2S”¢CO”¢HC1µČŃõ»Æ£¬³£ÓĆÓŚ¶ØĮæ²ā¶ØCOµÄŗ¬Į攣ŅŃÖŖ£ŗ
2I2(s)+5O2(g)£½2I2O5(s) ”÷H£½£75.56 kJ”¤mol£1
2CO(g)+O2(g)£½2CO2(g) ”÷H£½£566.0 kJ”¤mol£1
Š“³öCO(g)ÓėI2O5(s)·“Ӧɜ³ÉI2(s)ŗĶCO2(g)µÄČČ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø2£©Ņ»¶ØĢõ¼žĻĀ£¬NO2ÓėSO2·“Ӧɜ³ÉSO3ŗĶNOĮ½ÖÖĘųĢå£ŗNO2(g)+SO2(g) SO3(g)+NO(g)½«Ģå»ż±ČĪŖ1”Ć2µÄNO2”¢SO2ĘųĢåÖĆÓŚĆܱÕČŻĘ÷ÖŠ·¢ÉśÉĻŹö·“Ó¦£¬ĻĀĮŠÄÜĖµĆ÷·“Ó¦“ļµ½Ę½ŗāדĢ¬µÄŹĒ
ӣ
SO3(g)+NO(g)½«Ģå»ż±ČĪŖ1”Ć2µÄNO2”¢SO2ĘųĢåÖĆÓŚĆܱÕČŻĘ÷ÖŠ·¢ÉśÉĻŹö·“Ó¦£¬ĻĀĮŠÄÜĖµĆ÷·“Ó¦“ļµ½Ę½ŗāדĢ¬µÄŹĒ
ӣ
a£®ĢåĻµŃ¹Ēæ±£³Ö²»±ä
b£®»ģŗĻĘųĢåŃÕÉ«±£³Ö²»±ä
c£®SO3ŗĶNOµÄĢå»ż±Č±£³Ö²»±ä
d£®ĆæĻūŗÄ1molSO2µÄĶ¬Ź±Éś³É1molNO
²āµĆÉĻŹö·“Ó¦Ę½ŗāŹ±NO2ÓėSO2Ģå»ż±ČĪŖ1”Ć6£¬ŌņĘ½ŗā³£ŹżK£½ ”£
£Ø3£©“ÓĶŃĻõ”¢ĶŃĮņŗóµÄŃĢĘųÖŠ»ńČ”¶žŃõ»ÆĢ¼£¬ÓƶžŃõ»ÆĢ¼ŗĻ³É¼×“¼ŹĒĢ¼¼õÅŵĊĀ·½Ļņ”£½«CO2×Ŗ»ÆĪŖ¼×“¼µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗCO2
(g)£«3H2(g) CH3OH(g)£«H2O(g) ”÷H3
CH3OH(g)£«H2O(g) ”÷H3
¢ŁČ”Īå·ŻµČĢåĢå»żCO2ŗĶH2µÄµÄ»ģŗĻĘųĢå £ØĪļÖŹµÄĮæÖ®±Č¾łĪŖ1£ŗ3£©£¬·Ö±š¼ÓČėĪĀ¶Č²»Ķ¬”¢ČŻ»żĻąĶ¬µÄŗćČŻĆܱÕČŻĘ÷ÖŠ£¬·¢ÉśÉĻŹö·“Ó¦£¬·“Ó¦ĻąĶ¬Ź±¼äŗ󣬲āµĆ¼×“¼µÄĢå»ż·ÖŹż¦Õ(CH3OH) Óė·“Ó¦ĪĀ¶ČTµÄ¹ŲĻµĒśĻßČēĶ¼ĖłŹ¾£¬ŌņÉĻŹöCO2×Ŗ»ÆĪŖ¼×“¼·“Ó¦µÄ”÷H3 0£ØĢī”°£¾”±”¢”°£¼”±»ņ”°£½”±£©”£
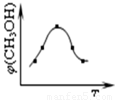
¢ŚŌŚČŻ»żĪŖ1LµÄŗćĪĀĆܱÕČŻĘ÷ÖŠ³äČė1molCO2ŗĶ3molH2£¬½ųŠŠÉĻŹö·“Ó¦”£²āµĆCO2ŗĶCH3OH(g)µÄÅضČĖꏱ¼ä±ä»ÆČēĻĀ×óĶ¼ĖłŹ¾”£ČōŌŚÉĻŹöĘ½ŗāĢåĻµÖŠŌŁ³ä0.5molCO2ŗĶ1.5molĖ®ÕōĘų£Ø±£³ÖĪĀ¶Č²»±ä£©£¬Ōņ“ĖĘ½ŗā½« ŅĘ¶Æ£ØĢī”°ĻņÕż·“Ó¦·½Ļņ”±”¢”°²»”±»ņ”°Äę·“Ó¦·½Ļņ”±£©”£
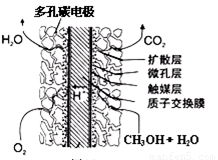
¢ŪÖ±½Ó¼×“¼Č¼ĮĻµē³Ų½į¹¹ČēÉĻÓŅĶ¼ĖłŹ¾”£Ę乤×÷Ź±øŗ¼«µē¼«·“Ó¦Ź½æɱķŹ¾ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğŗÓÄĻŹ”Ö£ÖŻŹŠøßČżµŚ¶ž“ĪÖŹĮæŌ¤²ā£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
ĄūÓĆĖłŃ§»Æѧ·“Ó¦ŌĄķ£¬½ā¾öŅŌĻĀĪŹĢā£ŗ
£Ø1£©Ä³ČÜŅŗŗ¬Į½ÖÖĻąĶ¬ĪļÖŹµÄĮæµÄČÜÖŹ£¬ĒŅĘäÖŠÖ»“ęŌŚOHŅ»”¢H£«”¢ ”¢ClŅ»ĖÄÖÖĄė×Ó£¬ĒŅc£Ø
”¢ClŅ»ĖÄÖÖĄė×Ó£¬ĒŅc£Ø £©>c£ØCl££©>c£ØOH££©>c£ØH£«£©£¬ŌņÕāĮ½ÖÖČÜÖŹŹĒ_____________”£
£©>c£ØCl££©>c£ØOH££©>c£ØH£«£©£¬ŌņÕāĮ½ÖÖČÜÖŹŹĒ_____________”£
£Ø2£©0£®1 mol”¤L£1µÄ°±Ė®Óė0£®05 mol”¤L£1µÄĻ”ĮņĖįµČĢå»ż»ģŗĻ£¬ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾»ģŗĻŗóČÜŅŗµÄĖį¼īŠŌ£ŗ______________________”£
£Ø3£©ŅŃÖŖ£ŗKsp£ØRX£©£½1£®8”Į10£10£¬Ksp£ØRY£©£½1£®5”Į10£16£¬Ksp£ØR2Z£©£½2£®0”Į10£12£¬ŌņÄŃČÜŃĪRX”¢RYŗĶR2ZµÄ±„ŗĶČÜŅŗÖŠ£¬R£«ÅضČÓɓ󵽊”µÄĖ³ŠņŹĒ_____________”£
£Ø4£©ŅŌŹÆÄ«µē¼«µē½ā100 mL 0£®1 mol”¤L£1CuSO4ČÜŅŗ”£ČōŃō¼«ÉĻ²śÉśĘųĢåµÄĪļÖŹµÄĮæĪŖ0£®01 mol£¬ŌņŅõ¼«ÉĻĪö³öCuµÄÖŹĮæĪŖ__________g”£
£Ø5£©Ļņ20 mLĮņĖįŗĶŃĪĖįµÄ»ģŗĻŅŗÖŠÖšµĪ¼ÓČėpH£½13µÄBa£ØOH£©2ČÜŅŗ£¬Éś³ÉBaSO4µÄĮæČēÓŅĶ¼ĖłŹ¾£¬BµćČÜŅŗµÄpH£½7£Ø¼ŁÉčĢå»żæÉŅŌÖ±½ÓĻą¼Ó£©£¬Ōņc£ØHCl£©£½_______mol”¤L£1.

£Ø6£©ŌŚĪĀ¶Č”¢ČŻ»żĻąĶ¬µÄ3øöĆܱÕČŻĘ÷ÖŠ£¬°“ĻĀ±ķĶ¶Čė·“Ó¦Īļ£¬·¢Éś·“Ó¦£ØH2£Øg£©£«I2£Øg£© 2HI£Øg£© ”÷H£½£14£®9 kJ”¤mol£1£©£¬ŌŚŗćĪĀ”¢ŗćČŻĢõ¼žĻĀ£¬²āµĆ·“Ó¦“ļµ½Ę½ŗāŹ±µÄŹż¾ŻČēĻĀ±ķ£ŗ
2HI£Øg£© ”÷H£½£14£®9 kJ”¤mol£1£©£¬ŌŚŗćĪĀ”¢ŗćČŻĢõ¼žĻĀ£¬²āµĆ·“Ó¦“ļµ½Ę½ŗāŹ±µÄŹż¾ŻČēĻĀ±ķ£ŗ

ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ_______________”£
A£® £«
£« £½1 B£®2
£½1 B£®2 £½
£½ C£®a£«b£½14£®9
D£®c1£½c2£½c3
C£®a£«b£½14£®9
D£®c1£½c2£½c3
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com