��2009?����ģ�⣩��ѧ��ȤС�����ͭ��ȡ����ͭ��������鷽�����о�������ͬѧ��ͭ��Ͷ��ʢ��ϡ����������У���������Ӧ�������������ײ�����������������Һ��������ɫ��������Һ�з�����Щ��˿����Ӧ�������Լӿ죮��ʱ���������ͬѧ����������£�
���飺Cu
CuO
CuSO
4 ���飺Cu
CuSO
4���飺Cu
Cu��NO
3��
2Cu��OH��
2CuSO
4���飺Cu
CuSO
4��1�������������һ����ѧ��Ӧ����ʽ��ʾΪ
2Cu+O2+2H2SO4=2CuSO4+2H2O
2Cu+O2+2H2SO4=2CuSO4+2H2O
��ʹ��Ӧ���Ŀ���ԭ����
�γ���ԭ���
�γ���ԭ���
���Ӹ÷�Ӧ����Һ����������H
2SO
4������ˮ�⣩��ȡ�ò�Ʒ�������������ӦΪ
����������
����������
��
��ȴ�ᾧ
��ȴ�ᾧ
��
���˲�ϴ�Ӿ���
���˲�ϴ�Ӿ���
��ȡ�ò�Ʒ��IJ������ʿ�ѭ��ʹ�ã�
��2���ɳ�����չ��ԭ��Ҫ��������ԭ����ѧ�����������ĵͣ���ԭ�������ʸߣ�������Ⱦ���������鷽���У��п�ѧ�Դ������
��
��
�鷽��������Ⱦ����
��
��
�鷽��������Ⱦ���������Ľϸߵ���
��
��
�鷽�������ѡ��
��
��
�鷽����ã�
����һ��ѧ��ȤС��ӻ�ѧ�ֲ��ϲ������ͭ500�������ϰ�����ʽ�ֽ⣺
CuSO
4CuO+SO
2��+SO
3��+O
2����������������ⶨ��Ӧ���ɵ�SO
2��SO
3��O
2�����ʵ�������������ȷ����������CuSO
4�ֽⷴӦ����ʽ�и����ʵĻ�ѧ����������������õ�����������ͼ��ʾ��
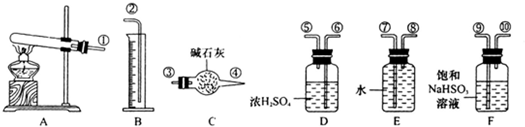
���������ݼ��йؽ�����£�
��ȷ��ȡ6.0g��ˮCuSO
4�������������ʱ���������������2.4g���۲����Ͳ��ˮ��������ó������ڱ�״���µ����Ϊ280mL���������ʱ��װ��F�е���Һֻ��NaHSO
4��NaHSO
3����װ�ô����ҵķ��������ӿ�����˳��Ϊ���٢��ޢݢۢܢ�ߢ�
�Իش��������⣺
��1��װ��F��������
����SO3���ų������ʵ�����SO2
����SO3���ų������ʵ�����SO2
��
��2��װ��D��������
����ˮ�֣�����SO2��O2
����ˮ�֣�����SO2��O2
��
��3��Ϊ���ٲ�����������������Ӧע��������У�
��װ����������¶�Ӧ�ָ������£�
��
������Ͳʹ��Һ����Eװ���е�Һ���ƽ
������Ͳʹ��Һ����Eװ���е�Һ���ƽ
��
��
������Ͳ��Һ�����ʱ��������Һ��İ�Һ����ƽ
������Ͳ��Һ�����ʱ��������Һ��İ�Һ����ƽ
��
��4��ͨ�����㣬�ƶϳ��������·�Ӧ�Ļ�ѧ����ʽ
3CuSO
43CuO+2SO
2��+SO
3��+O
2��
3CuSO
43CuO+2SO
2��+SO
3��+O
2��
��

 ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�

