
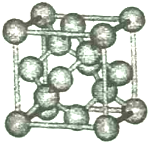
 2014��12��6���й��������������ձ�������ѧ��ѧ��ȷ���������·��ֵľ��и߶Ȼ��ԵĹ軯��SiC2N��SiC3N���ӣ������о�������������ѧ�����Ǽʽ�����Ѱ����ط��ӣ��ش��������⣺
2014��12��6���й��������������ձ�������ѧ��ѧ��ȷ���������·��ֵľ��и߶Ȼ��ԵĹ軯��SiC2N��SiC3N���ӣ������о�������������ѧ�����Ǽʽ�����Ѱ����ط��ӣ��ش��������⣺���� ��1��Siԭ�Ӻ�����14�����ӣ����ݹ���ԭ����д���������Ų�ʽ��Siԭ��3p�ܼ��ϵ���δ�ɶԣ�
��2�����ݼ۲���ӶԻ�������ȷ�������ռ乹�ͼ�Siԭ���ӻ���ʽ��������Siԭ�Ӽ۲���ӶԸ�����4�Ҳ����µ��Ӷԣ�
��3��ԭ�Ӿ����۷е�ϸߡ����Ӿ����۷е�ϵͣ�ԭ�Ӹ�����ȡ��۵�������ȵ�����Ϊ�ȵ����壻
��4�����۵���ֻ���Ҽ������������к���һ���Ҽ��������м���
��5���þ�����Siԭ�Ӹ���=4+8��$\frac{1}{8}$+6��$\frac{1}{2}$=8��ÿ��Siԭ�Ӻ���Si-Si������=4��$\frac{1}{2}$=2���þ������ⳤΪ0.543nm=5.43��10-6cm���������=��5.43��10-6cm��3���ܶ�=$\frac{m}{V}$��
��� �⣺��1��Siԭ�Ӻ�����14�����ӣ����ݹ���ԭ����д���������Ų�ʽΪ1s22s22p63s23p2��Siԭ��3p�ܼ��ϵ���δ�ɶԣ�������δ�ɶԵ��ӣ��ʴ�Ϊ��1s22s22p63s23p2��2��
��2��������Siԭ�Ӽ۲���ӶԸ�����4�Ҳ����µ��Ӷԣ�������ռ乹��Ϊ��������ṹ��Siԭ�Ӳ���sp3�ӻ���
�ʴ�Ϊ���������壻sp3��
��3��ԭ�Ӿ����۷е�ϸߡ����Ӿ����۷е�ϵͣ����ݾ����۷е�֪��Si����ԭ�Ӿ��塢SiCl4�ľ�������Ϊ���Ӿ��壻ԭ�Ӹ�����ȡ��۵�������ȵ�����Ϊ�ȵ����壬SiCl4��Ϊ�ȵ������һ�ַ���CCl4���ʴ�Ϊ��ԭ�Ӿ��壻���Ӿ��壻CCl4��
��4�����۵���ֻ���Ҽ������������к���һ���Ҽ��������м���N��Si-C��C-Si��N�ЦҼ����м������ֱ���5��6�����Զ���֮��Ϊ5��6���ʴ�Ϊ��5��6��
��5���þ�����Siԭ�Ӹ���=4+8��$\frac{1}{8}$+6��$\frac{1}{2}$=8��ÿ��Siԭ�Ӻ���Si-Si������=4��$\frac{1}{2}$=2������1mol����Si-Si��Ϊ2mol��
�þ������ⳤΪ0.543nm=5.43��10-6cm���������=��5.43��10-6cm��3���ܶ�=$\frac{m}{V}$=$\frac{\frac{28}{{N}_{A}}��8}{V}$=$\frac{224}{��5.43��1{0}^{-6}��^{3}{N}_{A}}$g•cm-3��
�ʴ�Ϊ��$\frac{224}{��5.43��1{0}^{-6}��^{3}{N}_{A}}$��
���� ���⿼����ۺϣ��漰�������㡢ԭ���ӻ������ռ乹���жϡ���������Ų���֪ʶ�㣬��Щ���Ǹ�Ƶ���㣬֪������ԭ�����۲���ӶԻ������۵�֪ʶ�㼴�ɽ���ѵ��ǣ�5���ļ��㣬ע�ⵥλ���㣬Ϊ�״��㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
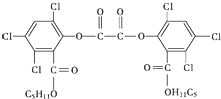 ��ħ�����������������ֳ����յ���Ⱦ����ħ��������ԭ��������H2O2��������������������������������ݸ�ӫ�����ʺ�㷢��ӫ�⣬���������CPPO���ṹ��ʽ��ͼ������˵������ȷ���ǣ�������
��ħ�����������������ֳ����յ���Ⱦ����ħ��������ԭ��������H2O2��������������������������������ݸ�ӫ�����ʺ�㷢��ӫ�⣬���������CPPO���ṹ��ʽ��ͼ������˵������ȷ���ǣ�������| A�� | CPPO������ˮ | |
| B�� | CPPO���ڷ�����Ҳ���ڸ߷��ӻ����� | |
| C�� | 1 mol CPPO��������ȫ��Ӧ����Ҫ10 mol H2 | |
| D�� | 1 mol CPPO��NaOHϡ��Һ��Ӧ�������DZ�������ԭ��ˮ�⣩���������4 mol NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʳ�׳����Ե�ԭ���ǣ�CH3COOH+H2O=CH3COO-+H3O+ | |
| B�� | ������Һ�ʼ��Ե�ԭ���ǣ�CO32-+2H2O?H2CO3+2OH- | |
| C�� | ��������������ⱥ��ʳ��ˮ�����ӷ���ʽ��Fe+2H2O $\frac{\underline{\;���\;}}{\;}$ Fe��OH��2+H2�� | |
| D�� | ��ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ��2H2��g��+O2��g��=2H2O��l������H=-571.6KJ•mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��ѧʽ | AgCl | Ag2CrO4 | CH3COOH | HClO | H2CO3 |
| Ksp��Ka | Ksp=1.8��10-10 | Ksp=9.0��10-12 | Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.1��10-7 Ka2=5.6��10-11 |
| A�� | ��ͬŨ��CH3COONa��NaClO�Ļ����Һ�У���������Ũ�ȵĴ�С��ϵ�ǣ�c��Na+����c��ClO-����c��CH3COO-����c��OH-����c��H+�� | |
| B�� | ����������Һ��ͨ������CO2�����ӷ���ʽΪ��2ClO-+CO2+H2O=CO32-+2HClO | |
| C�� | ��0.1 mol•L-1CH3COOH��Һ�еμ�NaOH��Һ����c��CH3COOH����c��CH3COO-��=5��9����ʱ��Һ��pH=5 | |
| D�� | ��Ũ�Ⱦ�Ϊ1.0��10-3 mol•L-1��KCl��K2CrO4�����Һ�еμ�1.0��10-3 mol•L-1��AgNO3��Һ��CrO42-���γɳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ļ��ԣ�H2O��H2S | B�� | �����ԣ�HF��Һ��HCl��Һ | ||
| C�� | ȼ���ȣ���H��C����s��ʯī������H��CO����g�� | D�� | ��ʴ�ԣ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ͷ��CuSO4��Һ�У�2Na+Cu2+=Cu+2Na+ | |
| B�� | ��NaHSO4��Һ�μӵ����з�̪��Ba��OH��2��Һ�У���Һ�ɺ�ɫ�����ɫ��Ba2++OH-+H++SO42-=BaSO4��+H2O | |
| C�� | ������������뵽NaAlO2��Һ�У�CH3COOH+AlO2-+H2O=CH3COO-+Al��OH��3�� | |
| D�� | ������Һ�м����������Ȼ�����Һ��S2-+2Fe3+=2Fe2++S�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
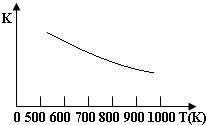 ����������������ѳ�ú���е�H2S����һ����ӦΪ��Fe3O4��s��+3H2S��g��+H2��g��?3FeS��s��+4H2O��g�����¶���ƽ�ⳣ���Ĺ�ϵ��ͼ��ʾ���Դ˷�Ӧԭ����������ȷ���ǣ�������
����������������ѳ�ú���е�H2S����һ����ӦΪ��Fe3O4��s��+3H2S��g��+H2��g��?3FeS��s��+4H2O��g�����¶���ƽ�ⳣ���Ĺ�ϵ��ͼ��ʾ���Դ˷�Ӧԭ����������ȷ���ǣ�������| A�� | H2S�ǻ�ԭ�� | B�� | �ѳ�H2S�ķ�Ӧ�Ƿ��ȷ�Ӧ | ||
| C�� | �¶�Խ��H2S���ѳ���Խ�� | D�� | ѹǿԽСH2S���ѳ���Խ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
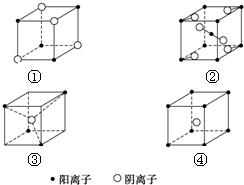
| A�� | ��MN ��MN2 ��MN2 ��MN | B�� | ��MN ��MN2 ��MN3 ��MN4 | ||
| C�� | ��MN2 ��MN2 ��MN2 ��MN | D�� | ��MN ��MN ��MN2 ��MN2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com