
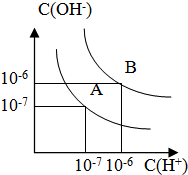
 ��֪ˮ��25���100��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���100��ʱ�������ƽ��������ͼ��ʾ������ ��1��������������Ũ�ȣ�����������������Ũ�ȣ�ˮ�����ӻ�����Kw=c��H+����c��OH-�������A���ߵ�Kw��Ȼ����ˮ�ĵ�����������ж�25��ʱˮ�ĵ���ƽ�����ߣ�
��2��������Һ��pH�������Һ�������ӡ�����������Ũ�ȣ�����ʽ���������������Һ��������Һ�������
��3����������B��Ӧ�¶���pH=5��˵����Һ��ʾ���ԣ���Ӧ�������ӹ���������
��� �⣺��1������A������Kw=c��H+����c��OH-��=10-7��10-7=10-14������B������c��H+��=c��OH-��=10-6 mol/L��Kw=c��H+��•c��OH-��=10-12��ˮ�ĵ���ʱ���ȹ��̣����ȴٽ����룬����A���ߴ���25��ʱˮ�ĵ���ƽ�����ߣ�
�ʴ�Ϊ��A��ˮ�ĵ��������ȹ��̣��¶ȵ�ʱ��ˮ�ĵ���̶�С��c��H+����c��OH-��С��
��2��100��ʱ���û����Һ��pH=6��Kw=c��H+����c��OH-��=10-6��10-6=10-12����Һ�����Լ����ǡ���кͣ���n��OH-��=n��H+������V��NaOH��•10-3 mol•L-1=V��H2SO4��•10-4 mol•L-1����V��NaOH����V��H2SO4��=1��10��
�ʴ�Ϊ��1��10��
��3��������B��Ӧ�¶��£���pH���ᣩ+pH���=12���ɵ��������Һ��c��H+��=c��OH-��������ǿ������Һ�������Ϻ���Һ�����ԣ��ֻ����Һ��pH=5�����������Ϻ���Һ�����ԣ�˵��H+��OH-��ȫ��Ӧ�������µ�H+�������������������HA�����ᣬ
�ʴ�Ϊ������B��Ӧ100�棬��ʱˮ�����ӻ�Ϊ10-12��HAΪ���ᣬHA�к�NaOH�����Һ�л�ʣ��϶��HA���ӣ��ɼ��������H+��ʹ��ҺpH=5��
���� ���⿼������Һ���������ҺpH�ļ��㣬��Ŀ�Ѷ��еȣ���ȷ������ʵĵ��뼰��Ӱ��Ϊ���ؼ���ע��������Һ���������ҺpH�Ĺ�ϵ�����㷽��������������ѧ�������Ӧ��������


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
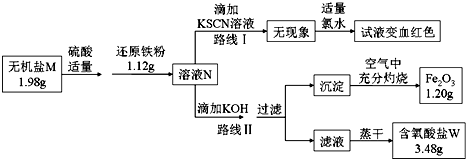
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ�� ��� | 0.01mol•L-1����KMnO4��Һ | 0.1mol•L-1 H2C2O4��Һ | ˮ | ��Ӧ�¶�/�� | ��Ӧʱ��/s |
| �� | 5.0mL | 5.0mL | 0 | 20 | 125 |
| �� | V1 | V2 | 2.0mL | 20 | 320 |
| �� | 5.0mL | 5.0mL | 0 | 50 | 30 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
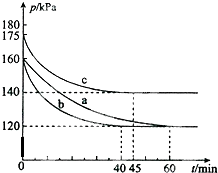 ��10L�ĺ����ܱ������У�������Ӧ��PCl3��g��+Cl2��g��?PCl5��g����H��0 ����ʼʱPCl3��g����Cl2��g����Ϊ0.2mol���ڲ�ͬ�����½���a��b��c����ʵ�飬ÿһ��ʵ�鶼���ں��º��������½��У���Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ������˵������ȷ���ǣ�������
��10L�ĺ����ܱ������У�������Ӧ��PCl3��g��+Cl2��g��?PCl5��g����H��0 ����ʼʱPCl3��g����Cl2��g����Ϊ0.2mol���ڲ�ͬ�����½���a��b��c����ʵ�飬ÿһ��ʵ�鶼���ں��º��������½��У���Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ������˵������ȷ���ǣ�������| A�� | ��ʵ��a��ȣ�ʵ��b�������¶ȣ�ʵ��c�����˴��� | |
| B�� | �ӷ�Ӧ��ʼ���մ�ƽ��ʱ��ʵ��b�Ļ�ѧ��Ӧ���ʦͣ�PCl5��=5��10-4mol/��L��min�� | |
| C�� | ʵ��c��ƽ��ʱ��PCl3��g����ת����Ϊ 60% | |
| D�� | ��ʵ��a�����£��÷�Ӧ��ƽ�ⳣ��K=100 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
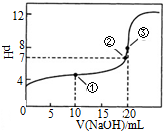 �����£���0.1000mol•L-1NaOH��Һ�ζ� 20.00mL0.1000mol•L-1CH3COOH��Һ�ζ�������ͼ����˵����ȷ���ǣ�������
�����£���0.1000mol•L-1NaOH��Һ�ζ� 20.00mL0.1000mol•L-1CH3COOH��Һ�ζ�������ͼ����˵����ȷ���ǣ�������| A�� | �����ʾ��Һ�У�c��CH3COOH��+c��CH3COO-����2c��Na+�� | |
| B�� | �����ʾ��Һ�У�c��Na+����c��OH-����c��CH3COO-����c��H+�� | |
| C�� | �����ʾ��Һ�У�c��CH3COO-����c��Na+�� | |
| D�� | �ζ������п��ܳ��֣�c��CH3COOH����c��CH3COO-����c��H+����c��Na+����c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��84������Һ����Ư��������Ϊ�����е�CO2������Һ�е�NaClO��Ӧ����HClO | |
| B�� | ���Ҵ����͡�����������������һ���������Ҵ����û��ȼ�ϵ���ֵҲ�����˸ı� | |
| C�� | �þ��������ϴ������ϩ���Ͽɼ��ٰ�ɫ��Ⱦ | |
| D�� | ˿����������Ԫ����ͬ�����ӽṹ��ͬ��������ʲ�ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
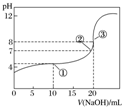
| A�� | ���к͵ζ����̣�������ʯ����ָʾ�� | |
| B�� | ͼ�е����ʾ��Һ��ˮ�ĵ���̶ȴ��ڵ����ʾ��Һ��ˮ�ĵ���̶� | |
| C�� | �ζ������е�ij�㣬����c��Na+����c��CH3COO-����c��H+����c��OH-���Ĺ�ϵ���� | |
| D�� | ͼ�е����ʾ��Һ�У�c��CH3COO-��=c��Na+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ʷ�����һ�������ڹ��ۼ� | |
| B�� | ��̬������һ���й��ۼ� | |
| C�� | �ڹ��ۻ�������һ���й��ۼ� | |
| D�� | �ɷǽ���Ԫ����ɵĻ������У�һ���������Ӽ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com